
1. Cvt 3146
2. Cvt-3146
3. Cvt3146
4. Lexiscan
1. 313348-27-5
2. Lexiscan
3. Rapiscan
4. Cvt-3146
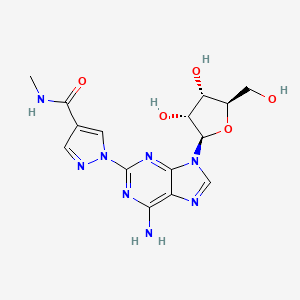
5. Regadenoson Anhydrous
6. 2-[4-[(methylamino)carbonyl]-1h-pyrazol-1-yl]adenosine
7. 7axv542lz4
8. Chembl317052
9. 1-(6-amino-9-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9h-purin-2-yl)-n-methyl-1h-pyrazole-4-carboxamide
10. Adenosine, 2-[4-[(methylamino)carbonyl]-1h-pyrazol-1-yl]-
11. Regadenoson Monohydrate
12. Lexiscan (tn)
13. Cvt 3146
14. Regadenoson [usan:inn]
15. Unii-7axv542lz4
16. Adenosine, 2-(4-((methylamino)carbonyl)-1h-pyrazol-1-yl)-
17. Regadenoson [mi]
18. Regadenoson [inn]
19. Regadenoson (usan/inn)
20. Regadenoson; Cvt-3146
21. Dsstox_cid_31501
22. Dsstox_rid_97386
23. Regadenoson [mart.]
24. Dsstox_gsid_57712
25. Schembl678893
26. Gtpl5596
27. Dtxsid4057712
28. Chebi:135613
29. Hms3886o21
30. Amy27715
31. Ex-a2148
32. Hy-a0168
33. Tox21_113668
34. Bdbm50119132
35. S5358
36. Zinc13818943
37. Akos026750593
38. Ccg-268525
39. Cs-5612
40. Cv-3146
41. Db06213
42. 1-(6-amino-9-beta-d-ribofuranosyl-9h-purin-2-yl)-n-methyl-1h-pyrazole-4-carboxamide
43. Ncgc00249892-01
44. (1-(9-(3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl)-6-aminopurin-2-yl)pyrazol-4-yl)-n-methylcarboxamide
45. Ac-35838
46. As-56292
47. B5904
48. Cas-313348-27-5
49. Cvt-3146;cvt3146;cvt 3146
50. D05711
51. 348r275
52. J-018384
53. Q7307897
54. (1-{9-[(4s,2r,3r,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6-aminopurin-2-yl}pyrazol-4-yl)-n-methylcarboxamide
55. 1-[6-amino-9-((2r,3r,4s,5r)-3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-9h-purin-2-yl]-1h-pyrazole-4-carboxylic Acid Methylamide
56. 1-{6-amino-9-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-9h-purin-2-yl}-n-methyl-1h-pyrazole-4-carboxamide
57. 6-amino-2-[4-(methylcarbamoyl)-1h-pyrazol-1-yl]purine-9-yl-beta-d-ribofuranoside;1-(6-amino-9-((2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yloxy)-9h-purin-2-yl)-n-methyl-1h-pyrazole-4-carboxamide
| Molecular Weight | 390.35 g/mol |
|---|---|
| Molecular Formula | C15H18N8O5 |
| XLogP3 | -1.5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 4 |
| Exact Mass | 390.14001570 g/mol |
| Monoisotopic Mass | 390.14001570 g/mol |
| Topological Polar Surface Area | 187 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 587 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lexiscan |
| PubMed Health | Regadenoson (Injection) |
| Drug Classes | Diagnostic Agent, Cardiac Function |
| Drug Label | Regadenoson is an A2A adenosine receptor agonist that is a coronary vasodilator [see Clinical Pharmacology (12.1)]. Regadenoson is chemically described as adenosine, 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]-, monohydrate. Its structural formula... |
| Active Ingredient | Regadenoson |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 0.4mg/5ml (0.08mg/ml) |
| Market Status | Prescription |
| Company | Astellas |
| 2 of 2 | |
|---|---|
| Drug Name | Lexiscan |
| PubMed Health | Regadenoson (Injection) |
| Drug Classes | Diagnostic Agent, Cardiac Function |
| Drug Label | Regadenoson is an A2A adenosine receptor agonist that is a coronary vasodilator [see Clinical Pharmacology (12.1)]. Regadenoson is chemically described as adenosine, 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]-, monohydrate. Its structural formula... |
| Active Ingredient | Regadenoson |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 0.4mg/5ml (0.08mg/ml) |
| Market Status | Prescription |
| Company | Astellas |
Diagnostic agent for radionuclide myocardial perfusion imaging (MPI)
FDA Label
This medicinal product is for diagnostic use only.
Rapiscan is a selective coronary vasodilator for use as a pharmacological stress agent for radionuclide myocardial perfusion imaging (MPI) in adult patients unable to undergo adequate exercise stress.
Regadenoson rapidly increases coronary blood flow (CBF) which is sustained for a short duration. Mean average peak velocity increased to greater than twice baseline by 30 seconds and decreased to less than twice the baseline level within 10 minutes. Myocardial uptake of the radiopharmaceutical is proportional to (CBF). Regadenoson increases blood flow in normal coronary arteries but not in stenotic (blocked) arteries. The significance of this finding is that stenotic arteries will take up less of the radiopharmaceutical than normal coronary arteries, resulting in a signal that is less intense in these areas.
Adenosine A2 Receptor Agonists
Compounds that selectively bind to and activate ADENOSINE A2 RECEPTORS. (See all compounds classified as Adenosine A2 Receptor Agonists.)
C01EB21
C01EB21
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01E - Other cardiac preparations
C01EB - Other cardiac preparations
C01EB21 - Regadenoson
Absorption
The pharmacokinetic profile of regadenoson is best described by a 3-compartment model. T max, injection = 1 to 3 minutes; Onset of pharmacodynamic response = 1 to 3 minutes; E max 12.3 ng/mL
Route of Elimination
58% of total regadenoson eliminate is via renal excretion
Volume of Distribution
Central compartment: 11.5 L; Steady state: 78.7 L
Clearance
Average plasma renal clearance = 450 mL/min. As this value is larger than the glomerular filtration rate, this suggests occurrence of renal tubular secretion.
The metabolism of regadenoson is unknown in humans. The cytochrome P450 enzyme system is not likely to be involved with the metabolism of regadenoson.
Initial phase: 2-4 minutes; Intermediate phase: 30 minutes (this phase coincides with a loss of the pharmacodynamic effect); Terminal phase: 2 hours
Regadenoson is an selective low-affinity (Ki= 1.3 M) A2A receptor agonist that mimics the effects of adenosine in causing coronary vasodilatation and increasing myocardial blood flow. It is a very weak agonist of the A1 adenosine receptor (Ki > 16.5 M). Furthermore, it has negligible affinity to A2B and A3 adenosine receptors. Regadenoson is undergoing trials for use in pharmacological stress tests. Adenosine slows conduction time through the A-V node, can interrupt the reentry pathways through the A-V node, and can restore normal sinus rhythm in patients with paroxysmal supraventricular tachycardia (PSVT), including PSVT associated with Wolff-Parkinson-White Syndrome.

LOOKING FOR A SUPPLIER?