
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. (13c3)-gs-5734
2. 13c3 Gs-5734
3. 13c3-gs-5734
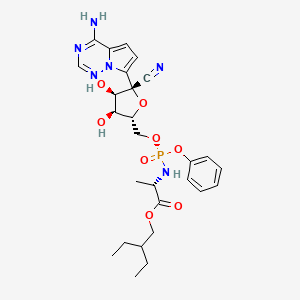
4. 2-ethylbutyl (2s)-2-(((2r, 3s, 4r, 5r)-5-(4-aminopyrrolo(2,1-f) (1,2,4)triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl) Methoxy)(phenoxy) Phosphoryl) Amino) Propanoate
5. Gs 5734
6. Gs-465124
7. Gs-5734
8. Gs-829143
9. L-alanine, N-((s)-hydroxyphenoxyphosphinyl)-, 2-ethylbutyl Ester, 6-ester With 2-c-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-d-altrononitrile
10. Veklury
1. 1809249-37-3
2. 3qki37eehe
3. Gs 5734
4. Gs 5734 [who-dd]
5. Gs-5734
6. Gs5734
7. Remdesivir [inn]
8. Remdesivir [usan]
9. Remdesivir [who-dd]
10. Remdesivirum
11. Unii-3qki37eehe
12. Veklury
13. 2-ethylbutyl (2s)-2-[[[(2r,3s,4r,5r)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate
14. 2-ethylbutyl (2s)-2-(((2r, 3s, 4r, 5r)-5-(4-aminopyrrolo(2,1-f) (1,2,4)triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl) Methoxy)(phenoxy) Phosphoryl) Amino) Propanoate
15. L-alanine, N-((s)-hydroxyphenoxyphosphinyl)-, 2-ethylbutyl Ester, 6-ester With 2-c-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-d-altrononitrile
16. 2-ethylbutyl (2s)-2-(((s)-(((2r,3s,4r,5r)-5-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
17. 2-ethylbutyl (2s)-2-{[(s)-{[(2r,3s,4r,5r)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl]methoxy}(phenoxy)phosphoryl]amino}propanoate
18. Veklury (tn)
19. Remdesivir [jan]
20. Remdesivir (jan/usan)
21. Remdesivir (gs-5734)
22. Chembl4065616
23. Remdesivir [orange Book]
24. Schembl17712225
25. Gtpl10715
26. Med.21724, Compound 178
27. Chebi:145994
28. Bdbm429505
29. Dtxsid701022537
30. Ex-a3265
31. Mfcd31657351
32. Nsc825151
33. S8932
34. At11308
35. Bcp24975-1
36. Db14761
37. Dt-0049
38. Nsc-825151
39. Compound 4b [pmid: 28124907]
40. Ncgc00686694-01
41. Ac-31297
42. Hy-104077
43. Cs-0028115
44. D11472
45. (2s)-2-{(2r,3s,4r,5r)-[5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxy-tetrahydro-furan-2-ylmethoxy]phenoxy-(s)-phosphorylamino}propionic Acid 2-ethyl-butyl Ester
46. (s)-2-ethylbutyl 2-(((s)-(((2r,3s,4r,5r)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
47. (s)-2-ethylbutyl 2-((s)-(((2r,3s,4r,5r)-5-(4-aminopyrrolo[1,2-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphorylamino)propanoate
48. 2-ethylbutyl (2s)-2-{[(s)-{[(2r,3s,4r,5r)-5-{4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl}-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy}(phenoxy)phosphoryl]amino}propanoate
49. 2-ethylbutyl N-((s)-(2-c-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-d-altrononitril-6-o-yl)phenoxyphosphoryl)-l-alaninate
50. 2-ethylbutyl N-[(s)-{[(2r,3s,4r,5r)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl]methoxy}(phenoxy)phosphoryl]-l-alaninate
| Molecular Weight | 602.6 g/mol |
|---|---|
| Molecular Formula | C27H35N6O8P |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 14 |
| Exact Mass | 602.22539909 g/mol |
| Monoisotopic Mass | 602.22539909 g/mol |
| Topological Polar Surface Area | 204 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Remdesivir is indicated for the treatment of adult and pediatric patients aged 12 years and over weighing at least 40 kg for coronavirus disease 2019 (COVID-19) infection requiring hospitalization. Under this indication, remdesivir should only be administered in a hospital or other healthcare setting capable of providing acute care comparable to an inpatient hospital setting. Remdesivir was originally granted FDA Emergency Use Authorization (EUA) on May 1, 2020, for use in adults and children with suspected or confirmed COVID-19 in a hospital setting with an SpO2 94%. Following FDA approval, this EUA was revised to cover hospitalized pediatric patients between 3.5 and 40 kg, as well as those under 12 years of age that weigh at least 3.5 kg, with suspected or laboratory-confirmed COVID-19. Under both the on-label and EUA indications, patients not needing invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) should be treated for 5 days (including the loading dose on day 1) and may be extended up to 10 days if they do not show improvement. Patients requiring invasive mechanical ventilation or ECMO should be treated for 10 days.
Veklury is indicated for the treatment of coronavirus disease 2019 (COVID 19) in:
- adults and paediatric patients (at least 4 weeks of age and weighing at least 3 kg) with pneumonia requiring supplemental oxygen (low- or high-flow oxygen or other non-invasive ventilation at start of treatment)
- adults and paediatric patients (weighing at least 40 kg) who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19
Remdesivir is a nucleoside analog used to inhibit the action of RNA polymerase. The duration of action is moderate, as it is given once daily. Due to much higher selectivity of mammalian DNA and RNA polymerases, including human mitochondrial RNA polymerase, for ATP over remdesivir triphosphate, remdesivir is not a significant inhibitor of these enzymes, which contributes to its overall tolerability and safety profile. Despite this, remdesivir carries risks for hypersensitivity reactions, including anaphylaxis and other infusion-related reactions, elevated transaminase levels, and potential decreased efficacy when combined with [hydroxychloroquine] or [chloroquine].
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB16 - Remdesivir
Absorption
Remdesivir is absorbed quickly; maximal plasma concentrations following a single 30-minute intravenous infusion are reached within 0.67-0.68 hours (Tmax). Repeated dosing yields a Cmax (coefficient of variation as a percent) of 2229 (19.2) ng/mL and an AUCtau of 1585 (16.6) ng\*h/mL. Remdesivir metabolite [GS-441524] has measured values: Tmax 1.51-2.00 hours, Cmax 145 (19.3) ng/mL, AUCtau 2229 (18.4) ng\*h/mL, and Ctrough 69.2 (18.2) ng/mL. Another metabolite, GS-704277, has measured values: Tmax 0.75 hours, Cmax 246 (33.9) ng/mL, AUCtau 462 (31.4) ng\*h/mL, and an undetermined Ctrough. A 10mg/kg intravenous dose given to cynomolgus monkeys distributes to the testes, epididymis, eyes, and brain within 4h.
Route of Elimination
Remdesivir is 74% eliminated in the urine and 18% eliminated in the feces. 49% of the recovered dose is in the form of the metabolite [GS-441524], and 10% is recovered as the unmetabolized parent compound. A small amount (0.5%) of the [GS-441524] metabolite is found in feces.
Volume of Distribution
Data regarding the volume of distribution of remdesivir is not readily available.
Clearance
Data regarding the clearance of remdesivir is not readily available.
Remdesivir is a phosphoramidate prodrug that must be metabolized within host cells to its triphosphate metabolite to be therapeutically active. Upon cell entry, remdesivir is presumed to undergo first esterase-mediated hydrolysis to a carboxylate form followed by cyclization to eject the phenoxide moiety and finally hydrolysis of the cyclic anhydride to yield the detectable alanine metabolite (GS-704277). The alanine metabolite is subsequently hydrolyzed to yield the monophosphate form of remdesivir, which is either hydrolyzed again to yield the bare nucleoside metabolite [GS-441524] or phosphorylated by cellular kinases to yield the active triphosphate form.
Remdesivir has an elimination half-life of 1 hour following a single 30-minute intravenous infusion. Under the same conditions, the elimination half-lives of the remdesivir metabolites [GS-441524] and GS-704277 are 27 hours and 1.3 hours, respectively. A 10mg/kg intravenous dose in non-human primates has a plasma half-life of 0.39h. The nucleoside triphosphate metabolite has a half-life of 14h in non-human primates. The nucleoside triphosphate metabolite has a half-life of approximately 20 hours in humans.
COVID-19 is caused by the positive-sense RNA virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Replication of the viral genome is a key step in the infectious cycle of RNA viruses, including those of the _Filoviridae_, _Paramyxoviridae_, _Pneumoviridae_, and _Coronaviridae_ families, and is carried out by viral RNA-dependent RNA polymerase (RdRp) enzymes or enzyme complexes. For both SARS-CoV and SARS-CoV-2, the RdRp comprises nsp7, nsp8, and nsp12 subunits under physiological conditions, although functional RdRp complexes can be reassembled _in vitro_ that incorporate only the nsp8 and nsp12 subunits, similar to the Middle East respiratory syndrome coronavirus (MERS-CoV). Remdesivir is a phosphoramidite prodrug of a 1'-cyano-substituted adenosine nucleotide analogue that competes with ATP for incorporation into newly synthesized viral RNA by the corresponding RdRp complex. Remdesivir enters cells before being cleaved to its monophosphate form through the action of either carboxylesterase 1 or cathepsin A; it is subsequently phosphorylated by undescribed kinases to yield its active triphosphate form remdesivir triphosphate (RDV-TP or GS-443902). RDV-TP is efficiently incorporated by the SARS-CoV-2 RdRp complex, with a 3.65-fold selectivity for RDV-TP over endogenous ATP. Unlike some nucleoside analogues, remdesivir provides a free 3'-hydroxyl group that allows for continued chain elongation. However, modelling and _in vitro_ experiments suggest that at _i_ + 4 (corresponding to the position for the incorporation of the fourth nucleotide following RDV-TP incorporation), the 1'-cyano group of remdesivir sterically clashes with Ser-861 of the RdRp, preventing further enzyme translocation and terminating replication at position _i_ + 3. This mechanism was essentially identical between SARS-CoV, SARS-CoV-2, and MERS-CoV, and genomic comparisons reveal that Ser-861 is conserved across alpha-, beta-, and deltacoronaviruses, suggesting remdesivir may possess broad antiviral activity. Considerations for the use of nucleotide analogues like remdesivir include the possible accumulation of resistance mutations. Excision of analogues through the 3'-5' exonuclease (ExoN) activity of replication complexes, mediated in SARS-CoV by the nsp14 subunit, is of possible concern. Murine hepatitis viruses (MHVs) engineered to lack ExoN activity are approximately 4-fold more susceptible to remdesivir, supporting the proposed mechanism of action. However, the relatively mild benefit of ExoN activity to remdesivir resistance is proposed to involve its delayed chain termination mechanism, whereby additional endogenous nucleotides are incorporated following RDV-TP. In addition, serial passage of MHV in increasing concentrations of the remdesivir parent molecule [GS-441524] led to the development of resistance mutations F476L and V553L, which maintain activity when transferred to SARS-CoV. However, these mutant viruses are less fit than wild-type in both competition assays and _in vivo_ in the absence of selective pressure. To date, no clinical data on SARS-CoV-2 resistance to remdesivir have been described.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
40
PharmaCompass offers a list of Remdesivir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Remdesivir manufacturer or Remdesivir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Remdesivir manufacturer or Remdesivir supplier.
PharmaCompass also assists you with knowing the Remdesivir API Price utilized in the formulation of products. Remdesivir API Price is not always fixed or binding as the Remdesivir Price is obtained through a variety of data sources. The Remdesivir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Remdesivir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Remdesivir, including repackagers and relabelers. The FDA regulates Remdesivir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Remdesivir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Remdesivir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Remdesivir supplier is an individual or a company that provides Remdesivir active pharmaceutical ingredient (API) or Remdesivir finished formulations upon request. The Remdesivir suppliers may include Remdesivir API manufacturers, exporters, distributors and traders.
click here to find a list of Remdesivir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Remdesivir DMF (Drug Master File) is a document detailing the whole manufacturing process of Remdesivir active pharmaceutical ingredient (API) in detail. Different forms of Remdesivir DMFs exist exist since differing nations have different regulations, such as Remdesivir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Remdesivir DMF submitted to regulatory agencies in the US is known as a USDMF. Remdesivir USDMF includes data on Remdesivir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Remdesivir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Remdesivir suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Remdesivir Drug Master File in Korea (Remdesivir KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Remdesivir. The MFDS reviews the Remdesivir KDMF as part of the drug registration process and uses the information provided in the Remdesivir KDMF to evaluate the safety and efficacy of the drug.
After submitting a Remdesivir KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Remdesivir API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Remdesivir suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Remdesivir as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Remdesivir API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Remdesivir as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Remdesivir and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Remdesivir NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Remdesivir suppliers with NDC on PharmaCompass.
Remdesivir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Remdesivir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Remdesivir GMP manufacturer or Remdesivir GMP API supplier for your needs.
A Remdesivir CoA (Certificate of Analysis) is a formal document that attests to Remdesivir's compliance with Remdesivir specifications and serves as a tool for batch-level quality control.
Remdesivir CoA mostly includes findings from lab analyses of a specific batch. For each Remdesivir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Remdesivir may be tested according to a variety of international standards, such as European Pharmacopoeia (Remdesivir EP), Remdesivir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Remdesivir USP).
