
Synopsis
Synopsis
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
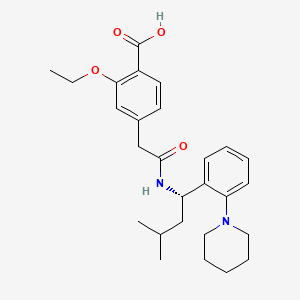

1. (+)-2-ethoxy-alpha-(((s)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic Acid
2. (+)-repaglinide
3. (-)-repaglinide
4. (s)-2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)-phenyl)butyl)amino)-2-oxoethyl)-benzoic Acid
5. 2-ethoxy-4-(2-(((1r)-3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)benzoic Acid
6. 2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)benzoic Acid
7. 2-ethoxy-n-(alpha-(2-methyl-1-propyl)-2-piperidinobenzyl)-4-carbamoylmethylbenzoic Acid
8. Ag-ee 388
9. Ag-ee 388 Zw
10. Ag-ee 623 Zw
11. Ag-ee 624zw
12. Ag-ee-623-zw
13. Ag-ee-623zw
14. Ag-ee-624zw
15. Agee-623zw
16. Gluconorm
17. Novonorm
18. Prandin
19. Repa-glinide
20. Repaglinide Impurity E
21. Repaglinide Related Compound E
22. Repaglinide, (+-)-isomer
1. 135062-02-1
2. Prandin
3. Novonorm
4. Gluconorm
5. Ag-ee 623 Zw
6. Ag-ee 388 Zw
7. Repaglinidum [inn-latin]
8. Repaglinida [inn-spanish]
9. Agee-623zw
10. (s)-2-ethoxy-4-(2-((3-methyl-1-(2-(piperidin-1-yl)phenyl)butyl)amino)-2-oxoethyl)benzoic Acid
11. C27h36n2o4
12. Ag-ee 623zw
13. Ag-ee-623zw
14. Ag-ee-623-zw
15. 2-ethoxy-4-[2-[[(1s)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic Acid
16. A10bx02
17. Nsc-759893
18. Chembl1272
19. (s)-(+)-2-ethoxy-4-[n-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic Acid
20. (s)-2-ethoxy-4-(2-(3-methyl-1-(2-(piperidin-1-yl)phenyl)butylamino)-2-oxoethyl)benzoic Acid
21. Chebi:8805
22. (-)-repaglinide
23. 668z8c33lu
24. 2-ethoxy-4-({[(1s)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]carbamoyl}methyl)benzoic Acid
25. 2-ethoxy-4-[2-({(1s)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl}amino)-2-oxoethyl]benzoic Acid
26. Ncgc00016978-01
27. Repaglinida
28. Repaglinidum
29. Repaglinide [usan]
30. Cas-135062-02-1
31. Actulin
32. Surepost
33. Reglin
34. Mfcd00906179
35. 2-ethoxy-4-(2-{[(1s)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino}-2-oxoethyl)benzoic Acid
36. Smr000466305
37. Prandin (tn)
38. Repaglinide (jan/usp/inn)
39. Unii-668z8c33lu
40. Ag-ee-623 Zw
41. Ag-ee-388
42. Repaglinide,(s)
43. Smp-508
44. Surepost (tn)
45. (s)-2-ethoxy-4-[2-[[3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]amino]-2-oxoethyl]benzoic Acid
46. Bjx
47. Repaglinide [usan:usp:inn:ban]
48. Repaglinide- Bio-x
49. Nn-623
50. (+)-2-ethoxy-alpha-(((s)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic Acid
51. 2-ethoxy-4-[2-[[(1s)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoic Acid
52. 111ge012
53. Repaglinide [mi]
54. Prestwick0_001046
55. Prestwick1_001046
56. Prestwick2_001046
57. Prestwick3_001046
58. Repaglinide [inn]
59. Repaglinide [jan]
60. Dsstox_cid_3552
61. Repaglinide [vandf]
62. (s)-2-ethoxy-4-(2-((methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-benzoic Acid
63. Dsstox_rid_77078
64. Repaglinide [mart.]
65. Dsstox_gsid_23552
66. Schembl16137
67. Bspbio_000972
68. Repaglinide [usp-rs]
69. Repaglinide [who-dd]
70. Mls000759407
71. Mls001076684
72. Mls001424111
73. Mls006011560
74. Bidd:gt0338
75. Spbio_002906
76. Repaglinide [ema Epar]
77. Bpbio1_001070
78. Gtpl6841
79. Dtxsid3023552
80. Repaglinide [orange Book]
81. Repaglinide For System Suitability
82. Hms1571a14
83. Hms2051n08
84. Hms2094c07
85. Hms2098a14
86. Hms2231m21
87. Hms3414d09
88. Hms3678d09
89. Hms3715a14
90. Pharmakon1600-01506035
91. Repaglinide [ep Monograph]
92. Repaglinide [usp Monograph]
93. Bcp04250
94. Zinc3798537
95. Tox21_110721
96. Ac-726
97. Bdbm50153520
98. Nsc759893
99. S1426
100. Stk629501
101. Akos005561792
102. Repaglinide, >=98% (hplc), Solid
103. Bs-1010
104. Ccg-101013
105. Cs-0979
106. Db00912
107. Nc00263
108. Nsc 759893
109. Repaglinide 100 Microg/ml In Methanol
110. Ncgc00016978-02
111. Ncgc00016978-04
112. Ncgc00016978-05
113. Benzoic Acid, 2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-, (s)-
114. Br164332
115. Hy-15209
116. Sbi-0206942.p001
117. Ab00514019
118. Am20090697
119. R0179
120. Sw197344-4
121. 62r021
122. C07670
123. D00594
124. F15001
125. Ab00514019-09
126. Ab00514019_10
127. Ab00514019_11
128. A806877
129. Sr-01000759404
130. Q-201663
131. Q2195995
132. Sr-01000759404-4
133. Brd-k82846253-001-03-0
134. Z1777729210
135. Repaglinide, European Pharmacopoeia (ep) Reference Standard
136. Repaglinide, United States Pharmacopeia (usp) Reference Standard
137. (s)-(+)-2-ethoxy-4-(2-oxo-2-[(alpha-isobutyl-2-piperidinobenzyl)amino]ethyl)-benzoic Acid
138. Repaglinide For System Suitability, European Pharmacopoeia (ep) Reference Standard
139. (+)-2-ethoxy-.alpha.-(((s)-.alpha.-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic Acid
140. (2s)-2-[[(2s)-2-[[(2s,3s)-2-[[(2s)-4-amino-2-[[(2s,3s)-2-[[(2s)-2-[[2-[[(2s)-2-[[(2s)-1-[(2s)-2-[[(2s)-2-amino-5-guanidino-pentanoyl]amino]-4-carboxy-butanoyl]pyrrolidine-2-carbonyl]amino]propanoyl]amino]acetyl]amino]-4-methyl-pentanoyl]amino]-3-methyl-pe;(s)-2-ethoxy-4-[2-[[3-methyl-1-[2-(1-piperidyl)phenyl]butyl]amino]-2-oxoethyl]benzoic Acid
141. (s)-2-ethoxy-4-(2-((3-methyl-1-(2-(piperidin-1-yl)phenyl)butyl)amino)-2-oxoethyl)benzoicacid
142. (s)-2-ethoxy-4-(2-(methyl-1-(2-(1-piperidinyl)phenyl)butylamino)-2-oxoethyl)-benzoic Acid
143. (s)-2-ethoxy-4-[2-[[[(1s)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoic Acid
144. (s)-2-ethoxy-4-[n-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonylmethyl]-benzoic Acid
145. 2-ethoxy-4-[2-[[(1s)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benz Oic Acid
146. 2-ethoxy-4-{[(s)-3-methyl-1-(2-piperidin-1-yl-phenyl)-butylcarbamoyl]-methyl}-benzoic Acid
147. Benzoic Acid, 2-ethoxy-4-[2-[[(1s)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]-
| Molecular Weight | 452.6 g/mol |
|---|---|
| Molecular Formula | C27H36N2O4 |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 452.26750763 g/mol |
| Monoisotopic Mass | 452.26750763 g/mol |
| Topological Polar Surface Area | 78.9 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 619 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Prandin |
| PubMed Health | Repaglinide (By mouth) |
| Drug Label | PRANDIN(repaglinide) is an oral blood glucose-lowering drug of the meglitinide clab used in the management of type 2 diabetes mellitus (also known as non-insulin dependent diabetes mellitus or NIDDM). Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1-... |
| Active Ingredient | Repaglinide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 2mg |
| Market Status | Prescription |
| Company | Novo Nordisk |
| 2 of 4 | |
|---|---|
| Drug Name | Repaglinide |
| PubMed Health | Repaglinide (By mouth) |
| Drug Label | PRANDIN(repaglinide) is an oral blood glucose-lowering drug of the meglitinide clab used in the management of type 2 diabetes mellitus (also known as non-insulin dependent diabetes mellitus or NIDDM). Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1-... |
| Active Ingredient | Repaglinide |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.5mg; 1mg; 2mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Aurobindo Pharma; Sun Pharm Inds; Actavis Totowa; Sandoz; Paddock |
| 3 of 4 | |
|---|---|
| Drug Name | Prandin |
| PubMed Health | Repaglinide (By mouth) |
| Drug Label | PRANDIN(repaglinide) is an oral blood glucose-lowering drug of the meglitinide clab used in the management of type 2 diabetes mellitus (also known as non-insulin dependent diabetes mellitus or NIDDM). Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1-... |
| Active Ingredient | Repaglinide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 2mg |
| Market Status | Prescription |
| Company | Novo Nordisk |
| 4 of 4 | |
|---|---|
| Drug Name | Repaglinide |
| PubMed Health | Repaglinide (By mouth) |
| Drug Label | PRANDIN(repaglinide) is an oral blood glucose-lowering drug of the meglitinide clab used in the management of type 2 diabetes mellitus (also known as non-insulin dependent diabetes mellitus or NIDDM). Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1-... |
| Active Ingredient | Repaglinide |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.5mg; 1mg; 2mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Aurobindo Pharma; Sun Pharm Inds; Actavis Totowa; Sandoz; Paddock |
As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
FDA Label
Repaglinide is indicated in patients with type-2 diabetes (non-insulin-dependent diabetes mellitus (NIDDM)) whose hyperglycaemia can no longer be controlled satisfactorily by diet, weight reduction and exercise.
Treatment should be initiated as an adjunct to diet and exercise to lower the blood glucose in relation to meals.
Repaglinide is indicated in patients with type-2 diabetes (non-insulin-dependent diabetes mellitus (NIDDM)) whose hyperglycaemia can no longer be controlled satisfactorily by diet, weight reduction and exercise. Repaglinide is also indicated in combination with metformin in type-2 diabetes patients who are not satisfactorily controlled on metformin alone.
Treatment should be initiated as an adjunct to diet and exercise to lower the blood glucose in relation to meals.
Repaglinide is indicated in patients with type-2 diabetes (non-insulin-dependent diabetes mellitus (NIDDM)) whose hyperglycaemia can no longer be controlled satisfactorily by diet, weight reduction and exercise.
Treatment should be initiated as an adjunct to diet and exercise to lower the blood glucose in relation to meals.
Repaglinide is indicated in patients with type-2 diabetes (non-insulin-dependent diabetes mellitus (NIDDM)) whose hyperglycaemia can no longer be controlled satisfactorily by diet, weight reduction and exercise. Repaglinide is also indicated in combination with metformin in type 2 diabetes patients who are not satisfactorily controlled on metformin alone.
Treatment should be initiated as an adjunct to diet and exercise to lower the blood glucose in relation to meals.
Repaglinide is indicated in patients with type-2 diabetes (non-insulin-dependent diabetes mellitus (NIDDM)) whose hyperglycaemia can no longer be controlled satisfactorily by diet, weight reduction and exercise. Repaglinide is also indicated in combination with metformin in type-2-diabetes patients who are not satisfactorily controlled on metformin alone.
Treatment should be initiated as an adjunct to diet and exercise to lower the blood glucose in relation to meals.
Repaglinide is indicated in patients with type-2 diabetes (non-insulin-dependent diabetes mellitus (NIDDM)) whose hyperglycaemia can no longer be controlled satisfactorily by diet, weight reduction and exercise. Repaglinide is also indicated in combination with metformin in type-2 diabetes patients who are not satisfactorily controlled on metformin alone.
Treatment should be initiated as an adjunct to diet and exercise to lower the blood glucose in relation to meals.
Insulin secretion by pancreatic β cells is partly controlled by cellular membrane potential. Membrane potential is regulated through an inverse relationship between the activity of cell membrane ATP-sensitive potassium channels (ABCC8) and extracellular glucose concentrations. Extracellular glucose enters the cell via GLUT2 (SLC2A2) transporters. Once inside the cell, glucose is metabolized to produce ATP. High concentrations of ATP inhibit ATP-sensitive potassium channels causing membrane depolarization. When extracellular glucose concentrations are low, ATP-sensitive potassium channels open causing membrane repolarization. High glucose concentrations cause ATP-sensitive potassium channels to close resulting in membrane depolarization and opening of L-type calcium channels. The influx of calcium ions stimulates calcium-dependent exocytosis of insulin granules. Repaglinide increases insulin release by inhibiting ATP-sensitive potassium channels in a glucose-dependent manner.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A10BX02
A10BX02
A10BX02
A10BX02
A10BX02
A10BX02
A10BX02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BX - Other blood glucose lowering drugs, excl. insulins
A10BX02 - Repaglinide
Absorption
Rapidly and completely absorbed following oral administration. Peak plasma concentrations are observed within 1 hour (range 0.5-1.4 hours). The absolute bioavailability is approximately 56%. Maximal biological effect is observed within 3-3.5 hours and plasma insulin levels remain elevated for 4-6 hours. When a single 2 mg dose of repaglinide is given to healthy subjects, the area under the curve (AUC) is 18.0 - 18.7 (ng/mL/h)^3.
Route of Elimination
90% eliminated in feces (<2% as unchanged drug), 8% in urine (0.1% as unchanged drug)
Volume of Distribution
31 L following IV administration in healthy individuals
Clearance
33-38 L/hour following IV administration
Repaglinide is rapidly metabolized via oxidation and dealkylation by cytochrome P450 3A4 and 2C9 to form the major dicarboxylic acid derivative (M2). Further oxidation produces the aromatic amine derivative (M1). Glucuronidation of the carboxylic acid group of repaglinide yields an acyl glucuronide (M7). Several other unidentified metabolites have been detected. Repaglinide metabolites to not possess appreciable hypoglycemic activity.
Repaglinide has known human metabolites that include 2-Hydroxy-4-[2-[[3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid, 2-ethoxy-4-[2-[[1-[2-(4-hydroxybutylamino)phenyl]-3-methylbutyl]amino]-2-oxoethyl]benzoic acid, 2-ethoxy-4-[2-[[3-hydroxy-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid, 3'-Hydroxy Repaglinide(Mixture of Diastereomers), and Repaglinide aromatic amine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
1 hour
Repaglinide activity is dependent on the presence functioning β cells and glucose. In contrast to sulfonylurea insulin secretatogogues, repaglinide has no effect on insulin release in the absence of glucose. Rather, it potentiates the effect of extracellular glucose on ATP-sensitive potassium channel and has little effect on insulin levels between meals and overnight. As such, repaglinide is more effective at reducing postprandial blood glucose levels than fasting blood glucose levels and requires a longer duration of therapy (approximately one month) before decreases in fasting blood glucose are observed. The insulinotropic effects of repaglinide are highest at intermediate glucose levels (3 to 10 mmol/L) and it does not increase insulin release already stimulated by high glucose concentrations (greater than 15 mmol/L). Repaglinide appears to be selective for pancreatic β cells and does not appear to affect skeletal or cardiac muscle or thyroid tissue.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?