

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
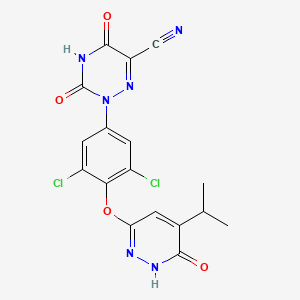

1. 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro(1,2,4)triazine-6-carbonitrile
2. Mgl-3196
1. Mgl-3196
2. 920509-32-6
3. Via-3196
4. Mgl 3196
5. 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
6. Resmetirom [usan]
7. Mgl3196
8. Re0v0t1es0
9. Chembl3261331
10. 1,2,4-triazine-6-carbonitrile, 2-(3,5-dichloro-4-((1,6-dihydro-5-(1-methylethyl)-6-oxo-3-pyridazinyl)oxy)phenyl)-2,3,4,5-tetrahydro-3,5-dioxo-
11. 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-(1,2,4)triazine-6-carbonitrile
12. 2-[3,5-dichloro-4-[(6-oxo-5-propan-2-yl-1h-pyridazin-3-yl)oxy]phenyl]-3,5-dioxo-1,2,4-triazine-6-carbonitrile
13. 2-[3,5-dichloro-4-[(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy]phenyl]-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
14. Unii-re0v0t1es0
15. Resmetirom [inn]
16. Resmetirom (usan/inn)
17. Resmetirom (mgl-3196)
18. Schembl2927241
19. Gtpl12026
20. Via3196
21. Hms3746a03
22. Amy16881
23. Bcp24624
24. Ex-a2477
25. Vlb50932
26. Bdbm50012905
27. Mfcd26142653
28. S6663
29. Who 10850
30. Zinc34842512
31. Akos032945066
32. Cs-6179
33. Db12914
34. Sb16825
35. Compound 53 [pmid: 24712661]
36. Ac-31458
37. Bs-17855
38. Hy-12216
39. Sy289460
40. Db-127167
41. C77150
42. D11602
43. Q27288071
44. 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro(1,2,4)triazine-6-carbonitrile
45. 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
46. 2-(4-(1,6-dihydro-5-isopropyl-6-oxopyridazin-3-yloxy)-3,5-dichlorophenyl)-2,3,4,5-tetrahydro-3,5-dioxo-1,2,4-triazine-6-carbonitrile
47. 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydro-pyridazin-3-yloxy)-phenyl]-3,5-dioxo-2,3,4,5-tetrahydro-[1,2,4]triazine-6-carbonitrile
| Molecular Weight | 435.2 g/mol |
|---|---|
| Molecular Formula | C17H12Cl2N6O4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 434.0297083 g/mol |
| Monoisotopic Mass | 434.0297083 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 878 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39990
Submission : 2024-05-27
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39990
Submission : 2024-05-27
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
About the Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experience,the company has ...
About the Company : Ami Lifesciences, established in 2006, is one of the fastest growing API manufacturing companies in India. Specializing in cardiovascular, anti-diabetic, CNS, and respiratory thera...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
About the Company : Since its inception in 2003, Seqens has grown to become a global leader in pharmaceutical solutions and specialty ingredients. Seqens supports its customers in developing, scaling ...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
About the Company : Egis is a member of the Servier Group. Egis’ products are manufactured at 3 production sites in Hungary, which are certified by EMA,FDA, ANVISA, PMDA ,KFDA. Egis sells its produc...
About the Company : Sintaho Pharmaceutical Co., Ltd., located in Chongqing, China, contributes to being the most dependable partner for global pharmaceutical companies. Sintaho specializes in chemosyn...
About the Company : Established in May 2012, Shandong Loncom Pharmaceutical operates as a fully owned subsidiary of Shandong Bestcomm Pharmaceutical Co., Ltd. Situated in the Qihe Economic Development...
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
About the Company : Specializing in natural & oncology APIs, we establish R&D and production platforms for new salt, crystal form & synthetic biology research. We cooperate with clients for IND, NDA, ...
About the Company : Royal pharma was started in 2007 – it’s a small scale Advanced Intermediates Manufacturing company. Facilities are in accordance to GMP standards. Currently working with Indian...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
