
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
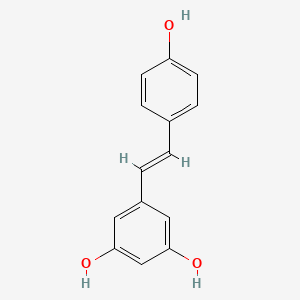

1. 3,4',5-stilbenetriol
2. 3,4',5-trihydroxystilbene
3. 3,5,4'-trihydroxystilbene
4. Cis Resveratrol
5. Cis-resveratrol
6. Resveratrol 3 Sulfate
7. Resveratrol, (z)-
8. Resveratrol-3-sulfate
9. Srt 501
10. Srt-501
11. Srt501
12. Trans Resveratrol
13. Trans Resveratrol 3 O Sulfate
14. Trans-resveratrol
15. Trans-resveratrol-3-o-sulfate
1. 501-36-0
2. Trans-resveratrol
3. 3,4',5-trihydroxystilbene
4. 3,5,4'-trihydroxystilbene
5. (e)-5-(4-hydroxystyryl)benzene-1,3-diol
6. (e)-resveratrol
7. Resvida
8. 5-[(e)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol
9. 3,4',5-stilbenetriol
10. 3,4',5-trihydroxy-trans-stilbene
11. Srt-501
12. Srt501
13. Srt 501
14. 3,5,4'-trihydroxy-trans-stilbene
15. Cuspidatin
16. Resveratol
17. Biofort
18. Polygonin
19. 5-[(1e)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol
20. Trans-3,4',5-trihydroxystilbene
21. Resveratrol P 5
22. (e)-5-(p-hydroxystyryl)resorcinol
23. 5-[(e)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol
24. Resveratrol(e)-form
25. 5-[2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol
26. Chebi:45713
27. Melinjo Resveratrol 20
28. C14h12o3
29. Srt 501m
30. 1,3-benzenediol, 5-[(1e)-2-(4-hydroxyphenyl)ethenyl]-
31. Chembl165
32. Nsc-327430
33. (e)-5-[2-(4-hydroxyphenyl)ethenyl]-1,3-benzendiol
34. 5-((1e)-2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol
35. Mls000069735
36. 1,3-benzenediol, 5-(2-(4-hydroxyphenyl)ethenyl)-, (e)-
37. Ca 1201
38. Trans-3,4',5 - Trihydroxystilbene
39. Bia 6-512
40. Bia-6-512
41. Nsc327430
42. Q369o8926l
43. Smr000058206
44. Dsstox_cid_11980
45. Dsstox_rid_78898
46. Dsstox_gsid_31980
47. 133294-37-8
48. Mfcd00133799
49. 3,4',5-trihydroxy-stilbene
50. Sr-01000000163
51. Trans-1,2-(3,4',5-trihydroxydiphenyl)ethylene
52. Nsc 327430
53. Chebi:27881
54. Ccris 8952
55. Unii-q369o8926l
56. Hsdb 7571
57. 3fts
58. 4jaz
59. 4qer
60. Resveratrol, E-
61. Resveratrol,(s)
62. Kuc104385n
63. Stilbene, 2f
64. Taxuschinensisirehd
65. Ncgc00015894-02
66. Cas-501-36-0
67. Stl
68. Prestwick_619
69. Resveratrol, Trans-
70. Ksc-10-164
71. Rm-1812
72. Opera_id_586
73. Resveratrol [mi]
74. Prestwick2_000508
75. Prestwick3_000508
76. Spectrum5_000552
77. Resveratrol [hsdb]
78. Resveratrol [inci]
79. R 5010
80. Resveratrol [vandf]
81. 1,3-benzenediol, 5-[(1z)-2-(4-hydroxyphenyl)ethenyl]-
82. Resveratrol [mart.]
83. Lopac0_001111
84. Regid_for_cid_6240
85. Schembl19425
86. Bspbio_000435
87. Bspbio_001114
88. Bspbio_003461
89. Resveratrol [who-dd]
90. Mls001055357
91. Mls001076538
92. Mls001424228
93. Mls002207121
94. Mls002222231
95. Spectrum1502223
96. Cu-01000001503-3
97. Bpbio1_000479
98. Cid_445154
99. Gtpl8741
100. Sgcut00007
101. Zinc6787
102. Resveratrol, Analytical Standard
103. Dtxsid4031980
104. Regid_for_cid_445154
105. Bdbm23926
106. Resveratrol, >=99% (hplc)
107. Amy5760
108. 2l98
109. Bcpp000091
110. Hms1362h15
111. Hms1569f17
112. Hms1792h15
113. Hms1921n04
114. Hms1990h15
115. Hms2052i09
116. Hms2096f17
117. Hms2232a18
118. Hms3263o04
119. Hms3403h15
120. Hms3412o14
121. Hms3649a20
122. Hms3676o14
123. Trans-resveratrol [usp-rs]
124. Act09778
125. Bcp01416
126. To_000079
127. Tox21_110257
128. Tox21_201374
129. Tox21_303376
130. Tox21_501111
131. Ac-727
132. Bbl028252
133. Ccg-38874
134. Lmpk13090005
135. S1396
136. Stl146386
137. Akos005720936
138. Tox21_110257_1
139. Cs-1050
140. Db02709
141. Ks-5047
142. Lp01111
143. Nc00349
144. Sdccgmls-0002998.p003
145. Sdccgsbi-0051080.p003
146. Idi1_002152
147. Ncgc00017352-05
148. Ncgc00017352-06
149. Ncgc00017352-07
150. Ncgc00017352-08
151. Ncgc00017352-09
152. Ncgc00017352-10
153. Ncgc00017352-11
154. Ncgc00017352-12
155. Ncgc00017352-13
156. Ncgc00017352-14
157. Ncgc00017352-15
158. Ncgc00017352-16
159. Ncgc00017352-17
160. Ncgc00017352-18
161. Ncgc00017352-19
162. Ncgc00017352-24
163. Ncgc00017352-31
164. Ncgc00017352-39
165. Ncgc00024003-00
166. Ncgc00024003-04
167. Ncgc00024003-05
168. Ncgc00024003-06
169. Ncgc00024003-07
170. Ncgc00024003-08
171. Ncgc00024003-09
172. Ncgc00024003-10
173. Ncgc00024003-11
174. Ncgc00024003-12
175. Ncgc00024003-13
176. Ncgc00024003-14
177. Ncgc00257465-01
178. Ncgc00258925-01
179. Ncgc00261796-01
180. As-12413
181. Hy-16561
182. Eu-0101111
183. N1848
184. R0071
185. Resveratrol, Vetec(tm) Reagent Grade, 98%
186. Sw196786-4
187. Trans [2,5,4'-trihydroxydiphenyl] Ethylene
188. 01r360
189. C03582
190. N88795
191. 5-[2-(4-hydroxyphenyl)vinyl]-1,3-benzenediol
192. Ab00052942-29
193. Ab00052942_31
194. Trans-resveratrol 100 Microg/ml In Acetonitrile
195. A827984
196. Q407329
197. 5-[(e)-2-(4-hydroxyphenyl)vinyl]-1,3-benzoldiol
198. Sr-01000000163-3
199. Sr-01000000163-4
200. Sr-01000000163-9
201. 5-[(e)-2-(4-hydroxyphenyl)ethenyl]benzol-1,3-diol
202. 5-[(e)-2-(4-hydroxyphenyl)vinyl]-1,3-benzenediol
203. 5[(e)-2-(4-hydroxyphenyl)-vinyl]benzene 1,3-diol
204. Brd-k25591257-001-01-2
205. Brd-k80738081-001-06-2
206. Brd-k80738081-001-07-0
207. Brd-k80738081-001-09-6
208. Brd-k80738081-001-10-4
209. Brd-k80738081-001-23-7
210. Sr-01000000163-10
211. Sr-01000000163-11
212. Sr-01000000163-16
213. (e)-1-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)ethene
214. (e)1-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)ethene
215. 5-[(1e)-2-(4-hydroxyphenyl)ethenyl]-1,3,benzenediol
216. Resveratrol, Certified Reference Material, Tracecert(r)
217. 1,3-benzenediol, 5-[(e)-2-(4-hydroxyphenyl)ethenyl]-
218. Resveratrol, European Pharmacopoeia (ep) Reference Standard
219. 5-((e)-2-(4-hydroxyphenyl)-ethenyl) Benzene-1,3 Diol
220. 533c1da0-4104-42b5-9d32-9265f40857e4
221. Trans-resveratrol, United States Pharmacopeia (usp) Reference Standard
222. 3,4',5-trihydroxy-trans-stilbene 5-[(1e)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol
223. (e)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol(e)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol
224. 31100-06-8
| Molecular Weight | 228.24 g/mol |
|---|---|
| Molecular Formula | C14H12O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 228.078644241 g/mol |
| Monoisotopic Mass | 228.078644241 g/mol |
| Topological Polar Surface Area | 60.7 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal; Anticarcinogenic Agents; Antimutagenic Agents; Antineoplastic Agents, Phytogenic; Antioxidants; Enzyme Inhibitors; Platelet Aggregation Inhibitors
NLM Medical Subjects Headings; 2014 MeSH; Available from, as of January 29, 2014: https://www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?mode=&term=resveratrol
/EXPL THER/ The antioxidant potency of three natural polyphenols, resveratrol, curcumin, and genistein, was compared by using the two human models: oxymodified with H2O2 and homocysteine (Hcy) G proteins in the postmortem frontal cortex (FC) membranes of age-matched control and Alzheimer's disease (AD) subjects; and Cu(2+)-induced oxidation of plasma low-density lipoproteins (LDL). In Co, 3-10 uM polyphenols dose-dependently depressed the G protein 25% stimulation induced by 10 uM H2O2 or 500 uM Hcy. Resveratrol revealed significantly higher antioxidativity than curcumin or genistein. In AD, the antioxidativity of polyphenols showed no significant differences. Polyphenols (1 uM) significantly increased the LDL oxidation lag time (oxyresistance) as compared with control, the effect of resveratrol being most potent. Due to the dual antioxidant mechanism, the investigated polyphenols, particularly resveratrol, should have preferences for the preventive-therapeutic use in age-related oxidative stress-based pathologies.
PMID:17404057 Jefremov V et al; Ann N Y Acad Sci 1095: 449-57 (2007)
/EXPL THER/ Arthritis, an inflammation of the joints, is usually a chronic disease that results from dysregulation of pro-inflammatory cytokines (e.g. tumor necrosis factor and interleukin-1beta) and pro-inflammatory enzymes that mediate the production of prostaglandins (e.g. cyclooxygenase-2) and leukotrienes (e.g. lipooxygenase), together with the expression of adhesion molecules and matrix metalloproteinases, and hyperproliferation of synovial fibroblasts. All of these factors are regulated by the activation of the transcription factor nuclear factor-kappaB. Thus, agents that suppress the expression of tumor necrosis factor-alpha, interleukin-1beta, cyclooxygenase-2, lipooxygenase, matrix metalloproteinases or adhesion molecules, or suppress the activation of NF-kappaB, all have potential for the treatment of arthritis. Numerous agents derived from plants can suppress these cell signaling intermediates, including curcumin (from turmeric), resveratrol (red grapes, cranberries and peanuts), tea polyphenols, genistein (soy), quercetin (onions), silymarin (artichoke), guggulsterone (guggul), boswellic acid (salai guggul) and withanolides (ashwagandha). Indeed, several preclinical and clinical studies suggest that these agents have potential for arthritis treatment. Although gold compounds are no longer employed for the treatment of arthritis, the large number of inexpensive natural products that can modulate inflammatory responses, but lack side effects, constitute 'goldmines' for the treatment of arthritis.
PMID:17475558 Khanna D et al; Curr Opin Pharmacol 7 (3): 344-51 (2007)
/EXPL THER/ Resveratrol, a red wine polyphenol, is known to protect against cardiovascular diseases and cancers, as well as to promote antiaging effects in numerous organisms. It also modulates pathomechanisms of debilitating neurological disorders, such as strokes, ischemia, and Huntington's disease. The role of resveratrol in Alzheimer's disease is still unclear, although some recent studies on red wine bioactive compounds suggest that resveratrol modulates multiple mechanisms of Alzheimer's disease pathology ...
PMID:16766037 Anekonda TS; Brain Res Rev 52 (2): 316-26 (2006)
For more Therapeutic Uses (Complete) data for RESVERATROL (12 total), please visit the HSDB record page.
Pregnant women and nursing mothers should avoid the use of resveratrol-containing supplements. They should also avoid the use of wine as a resveratrol source. Purple grape juice is a good and safe source of resveratrol, as well as other polyphenolic antioxidants.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 545
Resveratrol is contraindicated in those hypersensitive to any component of resveratrol-containing product.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 545
Being investigated for the treatment of Herpes labialis infections (cold sores).
Resveratrol, a phytoalexin, has been found to inhibit herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) replication in a dose-dependent, reversible manner, although this is only one of its many pharmaceutical properties. In some countries where there is higher consumption of red wine, there appears to be a lower incidence of heart disease. Other benefits of resveratrol include its anti-inflammatory and antioxidant effects. In preclinical studies, Resveratrol has been found to have potential anticancer properties.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Absorption
High absorption but very low bioavailability.
... Glycosylated resveratrol is more stable and more soluble and readily absorbed in the human gastrointestinal tract ... In humans, following its absorption, it is readily metabolized in the liver ... to water-soluble trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-O-sulfate, accounting for its predominant urine excretion ... Compared to other known polyphenols, such as quercetin and catechin, trans-resveratrol is well absorbed much more efficiently following oral administration to humans ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123
... Resveratrol is absorbed from the gastrointestinal tract following its ingestion ...
Thomson Healthcare. PDR for Nutritional Supplements. Thomson Health Care Inc. Montvale, NJ. p.399 (2001)
... Preclinical studies in rats, using HPLC methods, have suggested that intragastric administration of 20 mg/kg trans-resveratrol generated peak values of 1.2 uM in plasma ... In a separate study, male rats treated with 300, 1000, and 3000 mg/kg body weight per day were reported to achieve plasma concentrations of 576, 991, and 2728 ng/mL, respectively, and whereas in females, it was 333, 704, and 1137 ng/mL ... A plasma concentration of approximately 1.1 ug/mL was determined to be approximately 5 uM ... A single oral administration of (14)C-trans-resveratrol to male Balb/c mice showed preferential binding of radio-labeled resveratrol in the stomach, liver, kidney, intestine, bile, and urine, and penetrated the tissues of the liver and kidney ... Both the parent compound and the Phase-II metabolites were also detected in these tissues ... In humans, 24.6% of the oral dose administered appeared in the urine, including metabolites ... whereas after intragastric administration to rodents, only 1.5% of resveratrol reached the plasma compartment ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123
In a human melanoma xenograft model ... The resveratrol content in the skin of these mice, measured 5 min after a bolus of 75 mg/kg introduced, was found to be 21 nmol/g and 4.67 nmol/g in glucuronide-conjugate forms. A measurable amount of resveratrol was found in the tumors, although it was less than the amount found in the skin ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123
For more Absorption, Distribution and Excretion (Complete) data for RESVERATROL (12 total), please visit the HSDB record page.
Hepatic. Rapidly metabolized and excreted.
... This study ... examined the absorption, bioavailability, and metabolism of (14)C-resveratrol after oral and iv doses in six human volunteers. The absorption of a dietary relevant 25-mg oral dose was at least 70%, with peak plasma levels of resveratrol and metabolites of 491 + or - 90 ng/mL (about 2 uM) and a plasma half-life of 9.2 + or - 0.6 hr. However, only trace amounts of unchanged resveratrol (<5 ng/mL) could be detected in plasma. Most of the oral dose was recovered in urine, and liquid chromatography/mass spectrometry analysis identified three metabolic pathways, ie, sulfate and glucuronic acid conjugation of the phenolic groups and, interestingly, hydrogenation of the aliphatic double bond, the latter likely produced by the intestinal microflora. Extremely rapid sulfate conjugation by the intestine/liver appears to be the rate-limiting step in resveratrol's bioavailability.
PMID:15333514 Walle T et al; Drug Metab Dispos 32 (12): 1377-82 (2004)
In plants, it mostly exists in glycosylated piceid forms (3-O-B-D-glucosides). Other minor conjugated forms containing 1 to 2 methyl groups (pterostilbene), a sulfate group (trans-resveratrol-3-sulfate) or a fatty acid have also been identified. Glycosylation is known to protect resveratrol from oxidative degradation, and glycosylated resveratrol is more stable ... In humans, following its absorption, it is readily metabolized in the liver by Phase-2 drug-metabolizing enzymes to water-soluble trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-O-sulfate, accounting for its predominant urine excretion ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123
... Resveratrol was metabolized in humans into two metabolites, which were characterized as resveratrol-3-O- and 4'-O-glucuronides ...
PMID:15349955 Wang LX et al; J Pharm Sci 93 (10): 2448-57 (2004)
... In two 8-week long feeding experiments with rats, a low-resveratrol diet containing 50 mg resveratrol per kg body weight (bw) and day and a high-resveratrol diet with 300 mg per kg bw and day were administered. ... The level of resveratrol and its metabolites in the feces, urine, plasma, liver, and kidneys was identified and quantitated by high-performance liquid chromatography-diode array detection (HPLC-DAD) using synthesized resveratrol conjugate standards. ... The formation of trans-resveratrol-3-sulfate, trans-resveratrol-4'-sulfate, trans-resveratrol-3,5-disulfate, trans-resveratrol-3,4'-disulfate, trans-resveratrol-3,4',5-trisulfate, trans-resveratrol-3-O-beta-D-glucuronide, and resveratrol aglycone was detected by HPLC analysis, depending on the biological material. ...
PMID:15779067 Wenzel E et al; Mol Nutr Food Res 49 (5): 482-94 (2005)
Resveratrol has known human metabolites that include Resveratrol 3-O-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Pharmacokinetics of trans-resveratrol in its aglycone (RES(AGL)) and glucuronide (RES(GLU)) forms were studied following intravenous (15 mg/kg i.v.) and oral (50 mg/kg p.o.) administration of trans-resveratrol in a solution of beta-cyclodextrin to intact rats ... After i.v. administration, plasma concentrations of RES(AGL) declined with a rapid elimination half-life (T(1/2), 0.13 hr), followed by sudden increases in plasma concentrations 4 to 8 hr after drug administration. These plasma concentrations resulted in a significant prolongation of the terminal elimination half-life of RES(AGL) (T(1/2TER), 1.31 hr). RES(AGL) and RES(GLU) also displayed sudden increases in plasma concentrations 4 to 8 hr after oral administration, with T(1/2TER) of 1.48 and 1.58 hr, respectively ...
PMID:12065739 Marier JF et al; J Pharmacol Exp Ther 302 (1): 369-73 (2002)
Resveratrol ... has a plasma half-life of 8 to 14 min; the metabolites /(trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-O-sulfate)/ have a plasma half-life of about 9.2 hours ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123
... A dietary relevant 25-mg oral dose /of (14)C-resveratrol in six human volunteers resulted in/ ... a plasma half-life of 9.2 + or - 0.6 hr ...
PMID:15333514 Walle T et al; Drug Metab Dispos 32 (12): 1377-82 (2004)
A 25-mg oral dose /of (14)C-resveratrol had/ a plasma half-life of 9.2 + or - 0.6 hr. ...
PMID:15333514 Walle T et al; Drug Metab Dispos 32 (12): 1377-82 (2004)
Trans-resveratrol half-life /in human plasma/ was 1-3 hr following single-doses and 2-5 hr following repeated dosing.
PMID:19194969 Almeida L et al; Mol Nutr Food Res 53 (Suppl 1): S7-15 (2009)
Resveratrol suppresses NF-kappaB (NF-kappaB) activation in HSV infected cells. Reports have indicated that HSV activates NF-kappaB during productive infection and this may be an essential aspect of its replication scheme [PMID: 9705914].
... Resveratrol appears to act as a mixed agonist/antagonist for estrogen receptors alpha and beta. It has been found to bind estrogen receptor beta and estrogen receptor alpha with comparable affinity but with 7000-fold lower affinity than estradiol. Resveratrol differs from other phytoestrogens, which bind estrogen receptor beta with higher affinity than they bind estrogen receptor alpha. Resveratrol also shows estradiol antagonistic behavior for estrogen receptor alpha with some estrogen receptors. It does not show estradiol antagonistic activity with estrogen receptor beta ...
Thomson Healthcare. PDR for Nutritional Supplements. Thomson Health Care Inc. Montvale, NJ. p.398 (2001)
The anti-proliferative effects of resveratrol were shown to be modulated by cell cycle regulatory proteins ... Resveratrol decreased the expression of cyclins D1 and D2, Cdk 2, 4 and 6, and proliferating cell nuclear antigen (PCNA) whereas p21WAF1/CIP1 was increased. Furthermore there was inhibited expression of anti-apoptotic proteins, such as survivin, and markers of tumor promotion, cyclooxygenase (COX)-2, and ornithine decarboxylase (ODC) were observed ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123
... In addition to its antioxidant scavenging of free radicals and modulating estrogen receptor (ER) activity ... resveratrol can interfere with an ERalpha-associated PI3K pathway, following a process that could be independent of the nuclear functions of the ERalpha ... and acts as an agonist for the cAMP/kinase-A system ... It also promotes the accumulation of growth inhibitory/pro-apoptotic ceramide ... and induction of quinone reductase (QR, a phase II detoxification enzyme) ... and induces caspase-independent apoptosis through Bcl-2 downregulation ... It has been shown to suppress Src tyrosine kinase activity ... nitric oxide generation, and the NFkappaB pathway ...
Athar M et al; Toxicol Appl Pharmacol 224 (3): 274-283 (2007). Available from, as of August 20, 2014: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2083123

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
87
PharmaCompass offers a list of Resveratrol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Resveratrol manufacturer or Resveratrol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Resveratrol manufacturer or Resveratrol supplier.
PharmaCompass also assists you with knowing the Resveratrol API Price utilized in the formulation of products. Resveratrol API Price is not always fixed or binding as the Resveratrol Price is obtained through a variety of data sources. The Resveratrol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Resveratrol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Resveratrol, including repackagers and relabelers. The FDA regulates Resveratrol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Resveratrol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Resveratrol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Resveratrol supplier is an individual or a company that provides Resveratrol active pharmaceutical ingredient (API) or Resveratrol finished formulations upon request. The Resveratrol suppliers may include Resveratrol API manufacturers, exporters, distributors and traders.
click here to find a list of Resveratrol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Resveratrol DMF (Drug Master File) is a document detailing the whole manufacturing process of Resveratrol active pharmaceutical ingredient (API) in detail. Different forms of Resveratrol DMFs exist exist since differing nations have different regulations, such as Resveratrol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Resveratrol DMF submitted to regulatory agencies in the US is known as a USDMF. Resveratrol USDMF includes data on Resveratrol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Resveratrol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Resveratrol suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Resveratrol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Resveratrol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Resveratrol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Resveratrol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Resveratrol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Resveratrol suppliers with NDC on PharmaCompass.
Resveratrol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Resveratrol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Resveratrol GMP manufacturer or Resveratrol GMP API supplier for your needs.
A Resveratrol CoA (Certificate of Analysis) is a formal document that attests to Resveratrol's compliance with Resveratrol specifications and serves as a tool for batch-level quality control.
Resveratrol CoA mostly includes findings from lab analyses of a specific batch. For each Resveratrol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Resveratrol may be tested according to a variety of international standards, such as European Pharmacopoeia (Resveratrol EP), Resveratrol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Resveratrol USP).
