
Synopsis
Synopsis
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
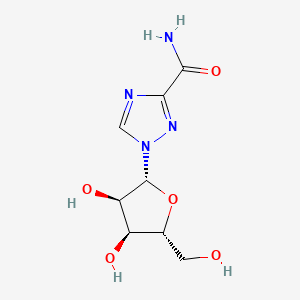

1. Icn 1229
2. Icn-1229
3. Icn1229
4. Rebetol
5. Ribamide
6. Ribamidil
7. Ribamidyl
8. Ribasphere
9. Ribovirin
10. Tribavirin
11. Vilona
12. Viramide
13. Virazide
14. Virazole
1. 36791-04-5
2. Tribavirin
3. Rebetol
4. Virazole
5. Copegus
6. Ribasphere
7. Vilona
8. Ribavirine
9. Viramid
10. Rtca
11. Cotronak
12. Ribamide
13. Ribamidil
14. Ribavirina
15. Ribavirinum
16. Ribavirine [inn-french]
17. Ribavirinum [inn-latin]
18. Icn-1229
19. Ribavirina [inn-spanish]
20. 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide
21. Ribavirin Mylan
22. Ribavirin Teva
23. 1-beta-d-ribofuranosyl-1h-1,2,4-triazole-3-carboxamide
24. Ribavirin Biopartners
25. Sch 18908
26. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1h-1,2,4-triazole-3-carboxamide
27. Rbv
28. Ribamidyl
29. Ribavirin (copegus)
30. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,4-triazole-3-carboxamide
31. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1h-1,2,4-triazole-3-carboxamide
32. Rebetron
33. Varazid
34. Nsc-163039
35. Ribavirin Capsules
36. 1h-1,2,4-triazole-3-carboxamide, 1-beta-d-ribofuranosyl-
37. Chebi:63580
38. 49717awg6k
39. Sch-18908
40. 1-(beta-d-ribofuranosyl)-1h-1,2,4-triazole-3-carboxamide
41. Rebretron
42. Virazid
43. Virazide
44. 1h-1,2,4-triazole-3-carboxamide, 1-.beta.-d-ribofuranosyl-
45. Ribav
46. Mfcd00058564
47. Dsstox_cid_3557
48. Dsstox_rid_77081
49. Dsstox_gsid_23557
50. Rtc
51. Ravanex
52. Ribacine
53. C-virin
54. Drg-0028
55. Ribasphere (tn)
56. Virazole (tn)
57. Smr000058315
58. Copegus (tn)
59. Rebetol (tn)
60. Hsdb 6513
61. Sr-01000076112
62. Brn 0892462
63. Ribavirin Teva Pharma B.v.
64. Unii-49717awg6k
65. Ribavirine;
66. Nsc163039
67. Ribavirin [usan:usp:inn:ban]
68. Ribavirin,(s)
69. Ncgc00015904-02
70. Ribavirin, Antiviral
71. Cas-36791-04-5
72. Rg-964
73. Ro-20-9963
74. Spectrum_001826
75. 4pb1
76. Ribavirin [inn]
77. Ribavirin [jan]
78. Ribavirin [mi]
79. Ribofluranosyl Carboxamide
80. Ribavirin [hsdb]
81. Ribavirin [usan]
82. Prestwick3_000993
83. Spectrum3_001876
84. Spectrum4_001252
85. Spectrum5_002075
86. Ribavirin [vandf]
87. R-964
88. Ribavirin [mart.]
89. R 9644
90. Ribavirin [usp-rs]
91. Ribavirin [who-dd]
92. Schembl3727
93. 1-.beta.-d-ribofuranosyl-1h-1,2,4-triazole-3-carboxamide
94. Chembl1643
95. Lopac0_001063
96. Bspbio_001085
97. Bspbio_003352
98. Kbiogr_001804
99. Kbioss_002331
100. Ribavirin [ema Epar]
101. Cid_37542
102. Mls000028486
103. Mls002222317
104. Divk1c_000782
105. Spectrum1503938
106. Ribavirin (jp17/usp/inn)
107. Bpbio1_001195
108. Gtpl6842
109. Ribavirin [orange Book]
110. 1,2,4-triazole-3-carboxamide, 1-beta-d-ribofuranosyl-
111. 1-(beta-d-ribofuranosyl)-1,2,4-triazole-3-carboxamide
112. 1-.beta.-d-ribofuranosyl-1,2,4-triazolo-3-carboxamide
113. Dtxsid8023557
114. Ribavirin [ep Monograph]
115. Hms502h04
116. Kbio1_000782
117. Kbio2_002328
118. Kbio2_004896
119. Kbio2_007464
120. Kbio3_002854
121. Ribavirin [usp Monograph]
122. Ninds_000782
123. Hms2090l15
124. Hms2094o09
125. Hms2098g07
126. Hms2232p07
127. Hms3263e08
128. Hms3715g07
129. Pharmakon1600-01503938
130. 66510-90-5
131. Hy-b0434
132. Zinc1035331
133. Tox21_110259
134. Tox21_200967
135. Tox21_501063
136. Bdbm50154375
137. Ccg-38985
138. Nsc758650
139. S2504
140. Ribavirin 100 Microg/ml In Methanol
141. Akos001715163
142. Tox21_110259_1
143. Db00811
144. Gs-3572
145. Lp01063
146. Nsc-758650
147. Sdccgsbi-0051033.p004
148. Idi1_000782
149. Smp1_000261
150. Ncgc00090726-01
151. Ncgc00090726-03
152. Ncgc00090726-04
153. Ncgc00090726-05
154. Ncgc00090726-06
155. Ncgc00090726-07
156. Ncgc00090726-08
157. Ncgc00090726-09
158. Ncgc00090726-12
159. Ncgc00090726-25
160. Ncgc00090726-30
161. Ncgc00258520-01
162. Ncgc00261748-01
163. 252269-50-4
164. As-34178
165. Bcp0726000138
166. Ribavirin Component Of Peginterferon
167. Sbi-0051033.p003
168. Ab00430481
169. Eu-0101063
170. R0077
171. En300-59237
172. D00423
173. Ab00430481-15
174. Ab00430481-16
175. Ab00430481_17
176. Ab00430481_18
177. 1-beta-ribofuranosyl-1,2,4-triazole-3-carboamide
178. 791r045
179. A823370
180. Q421862
181. Ribavirin 100 Microg/ml In Acetonitrile:methanol
182. Sr-01000721904
183. 1-b-d-ribofuranosyl-1,2,4-triazole-3-carboxamide
184. 1-ss-d-ribofuranosyl-1,2,4-triazole-3-carboxamide
185. Sr-01000076112-2
186. Sr-01000076112-3
187. Sr-01000076112-4
188. Sr-01000721904-2
189. Brd-k60369935-001-02-7
190. Brd-k60369935-001-18-3
191. Sr-01000076112-11
192. 1-?-d-ribofuranosyl-1h-1,2,4-triazole-3-carboxamide
193. Ribavirin, British Pharmacopoeia (bp) Reference Standard
194. Z1522567185
195. 1-(beta -d-ribofuranosyl)-1,2,4-triazole-3-carboxamide
196. Ribavirin, European Pharmacopoeia (ep) Reference Standard
197. Ribavirin, United States Pharmacopeia (usp) Reference Standard
198. Ribavirin, Pharmaceutical Secondary Standard; Certified Reference Material
199. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,2,4-triazole-3-carboxamide
200. 1-[(2r,3s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,2,4-triazole-3-carboxamide
| Molecular Weight | 244.20 g/mol |
|---|---|
| Molecular Formula | C8H12N4O5 |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 244.08076950 g/mol |
| Monoisotopic Mass | 244.08076950 g/mol |
| Topological Polar Surface Area | 144 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 304 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Copegus |
| PubMed Health | Ribavirin |
| Drug Classes | Antiviral |
| Drug Label | COPEGUS, ribavirin, is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1--D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide and has the following structural formula:The empirical formula of ribavirin is C8H12N4O5 and... |
| Active Ingredient | Ribavirin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Roche |
| 2 of 10 | |
|---|---|
| Drug Name | Rebetol |
| PubMed Health | Ribavirin |
| Drug Classes | Antiviral |
| Drug Label | REBETOL (ribavirin), is a synthetic nucleoside analogue (purine analogue). The chemical name of ribavirin is 1--D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide and has the following structural formula (see Figure 1).Figure 1: Structural FormulaRiba... |
| Active Ingredient | Ribavirin |
| Dosage Form | Capsule; Solution |
| Route | Oral |
| Strength | 200mg; 40mg/ml; 200mg **indicated for use and comarketed with interferon alfa-2b, recombinant (intron a), as rebetron combination therapy** |
| Market Status | Prescription |
| Company | Merck Sharp Dohme; Schering |
| 3 of 10 | |
|---|---|
| Drug Name | Ribasphere |
| PubMed Health | Ribavirin |
| Drug Classes | Antiviral |
| Drug Label | RIBASPHERE (ribavirin, USP), is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1-and has the following structural formula:The molecular formula of ribavirin is C8H12N4O5 and the molecular weight is 244.2. Ribavirin i... |
| Active Ingredient | Ribavirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Three Rivers Pharms |
| 4 of 10 | |
|---|---|
| Drug Name | Ribavirin |
| Drug Label | RIBASPHERE (ribavirin, USP), is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1-and has the following structural formula:The molecular formula of ribavirin is C8H12N4O5 and the molecular weight is 244.2. Ribavirin i... |
| Active Ingredient | Ribavirin |
| Dosage Form | Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 200mg; 600mg; 500mg; 400mg; 40mg/ml |
| Market Status | Tentative Approval; Prescription |
| Company | Teva; Aurobindo Pharma; Sandoz; Teva Pharms; Zydus Pharms Usa; Three Rivers Pharms |
| 5 of 10 | |
|---|---|
| Drug Name | Virazole |
| Drug Label | VIRAZOLE is a brand name for ribavirin, a synthetic nucleoside with antiviral activity. VIRAZOLE for inhalation solution is a sterile, lyophilized powder to be reconstituted for aerosol administration. Each 100 mL glass vial contains 6 grams of rib... |
| Active Ingredient | Ribavirin |
| Dosage Form | For solution |
| Route | Inhalation |
| Strength | 6gm/vial |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 6 of 10 | |
|---|---|
| Drug Name | Copegus |
| PubMed Health | Ribavirin |
| Drug Classes | Antiviral |
| Drug Label | COPEGUS, ribavirin, is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1--D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide and has the following structural formula:The empirical formula of ribavirin is C8H12N4O5 and... |
| Active Ingredient | Ribavirin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Roche |
| 7 of 10 | |
|---|---|
| Drug Name | Rebetol |
| PubMed Health | Ribavirin |
| Drug Classes | Antiviral |
| Drug Label | REBETOL (ribavirin), is a synthetic nucleoside analogue (purine analogue). The chemical name of ribavirin is 1--D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide and has the following structural formula (see Figure 1).Figure 1: Structural FormulaRiba... |
| Active Ingredient | Ribavirin |
| Dosage Form | Capsule; Solution |
| Route | Oral |
| Strength | 200mg; 40mg/ml; 200mg **indicated for use and comarketed with interferon alfa-2b, recombinant (intron a), as rebetron combination therapy** |
| Market Status | Prescription |
| Company | Merck Sharp Dohme; Schering |
| 8 of 10 | |
|---|---|
| Drug Name | Ribasphere |
| PubMed Health | Ribavirin |
| Drug Classes | Antiviral |
| Drug Label | RIBASPHERE (ribavirin, USP), is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1-and has the following structural formula:The molecular formula of ribavirin is C8H12N4O5 and the molecular weight is 244.2. Ribavirin i... |
| Active Ingredient | Ribavirin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Three Rivers Pharms |
| 9 of 10 | |
|---|---|
| Drug Name | Ribavirin |
| Drug Label | RIBASPHERE (ribavirin, USP), is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1-and has the following structural formula:The molecular formula of ribavirin is C8H12N4O5 and the molecular weight is 244.2. Ribavirin i... |
| Active Ingredient | Ribavirin |
| Dosage Form | Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 200mg; 600mg; 500mg; 400mg; 40mg/ml |
| Market Status | Tentative Approval; Prescription |
| Company | Teva; Aurobindo Pharma; Sandoz; Teva Pharms; Zydus Pharms Usa; Three Rivers Pharms |
| 10 of 10 | |
|---|---|
| Drug Name | Virazole |
| Drug Label | VIRAZOLE is a brand name for ribavirin, a synthetic nucleoside with antiviral activity. VIRAZOLE for inhalation solution is a sterile, lyophilized powder to be reconstituted for aerosol administration. Each 100 mL glass vial contains 6 grams of rib... |
| Active Ingredient | Ribavirin |
| Dosage Form | For solution |
| Route | Inhalation |
| Strength | 6gm/vial |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
Antimetabolites; Antiviral Agents
National Library of Medicine, SIS; ChemIDplus Record for Ribavirin (36791-04-5), MESH Heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Antiviral
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1470
Oral and intravenous ribavirin are used in the treatment of Lassa fever and as post-exposure prophylaxis in contacts at hgh risk. It may be similarly effective with other viral hemorrhagic fevers, including hemorrhagic fever with renal syndrome, Crimean-Congo hemorrhagic fever, and Rift Valley fever. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2584
Ribavirin inhalation solution is used as a secondary agent in the treatment of influenza A and B in young adults when treatment is started early (eg, within 24 hours of initial symptoms) in the course of the disease. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2584
Ribavirin inhalation solution is for the treatment of severe lower respiratory tract infections (including bronchiolitis and pneumonia) caused by respiratory syncytial virus (RSV) in hospitalized infants and young children who are at high risk for severe or complicated RSV infection; this category includes premature infants and infants with structural or physiologic cardiopulmonary disorder, bronchopulmonary dysplasia, immunodeficiency, or imminent respiratory failure. Ribavirin is indicated in the treatment of RSV infections in infants requiring mechanical ventilator assistance. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2584
FDA Pregnancy Risk Category: X /CONTRAINDICATED IN PREGNANCY. Studies in animals or humans, or investigational or post-marketing reports, have demonstrated positive evidence of fetal abnormalities or risk which clearly outweights any possible benefit to the patient./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2585
Evidence of disease progression, such as hepatic inflammation and fibrosis, as well as prognostic factors for response. HCV genotype and viral load, should be considered when deciding to treat a pediatric patient. The benefits of treatment should be weighed against the safety findings observed for pediatric patients in clinical trials.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2585
Worsening of respiratory function has occurred, sometimes suddenly, during ribavirin inhalation therapy in infants with RSV infections or in adults with chronic obstructive pulmonary disease (COPD) or asthma. In infants with underlying life-threatening conditions, inhalation of the drug has been associated with aggravation and worsening of respiratory function, apnea, and physical dependence on assisted respiration. In adults with COPD or asthma, therapy with the drug frequently has been associated with deterioration in pulmonary function, and dyspnea and chest soreness have occurred in several adults with asthma. Minor pulmonary function abnormalities have also been observed in healthy adults receiving ribavirin inhalation. Bronchospasm, pulmonary edema, hypoventilation, cyanosis, dyspnea, bacterial pneumonia, pneumothorax, apnea, atelectasis, and ventilator dependence also have been associated with ribavirin inhalation therapy. Several deaths that were characterized as possibly related to ribavirin inhalation therapy by the treating physician occurred in infants who experienced worsening respiratory status related to bronchospasm while receiving the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 805
Rash, erythema of the eyelids, and conjunctivitis have occurred in patients receiving ribavirin inhalation therapy. These effects usually resolve within hours after ribavirin therapy is discontinued. In addition, hearing disorders (e.g., hearing loss, tinnitus), vertigo, hypertriglyceridemia, and fatal and nonfatal pancreatitis have been observed in patients receiving ribavirin in conjunction with interferon alfa-2b.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 806
For more Drug Warnings (Complete) data for RIBAVIRIN (23 total), please visit the HSDB record page.
Indicated for the treatment of chronic Hepatitis C virus (HCV) infection in combination with other antiviral agents with the intent to cure or achieve a sustained virologic response (SVR). Typically added to improve SVR and reduce relapse rates. The addition of ribavirin in Technivie therapy indicated for treating HCV genotype 1a and 4 infections is recommended in patients with or without cirrhosis. Resistance: viral genetic determinants resulting in variable response to ribavirin therapy has not been yet determined.
FDA Label
Rebetol is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adults.
Rebetol is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) for paediatric patients (children 3 years of age and older and adolescents) not previously treated and without liver decompensation.
Ribavirin Mylan is indicated for the treatment of chronic hepatitis C and must only be used as part of a combination regimen with interferon alfa-2b (adults, children (three years of age and older) and adolescents). Ribavirin monotherapy must not be used.
There is no safety or efficacy information on the use of ribavirin with other forms of interferon (i. e. not alfa-2b).
Please refer also to the interferon alfa-2b summary of product characteristics (SmPC) for prescribing information particular to that product.
* Nave patients:
Adult patients
Ribavirin Mylan is indicated, in combination with interferon alfa-2b, for the treatment of adult patients with all types of chronic hepatitis C except genotype 1, not previously treated, without liver decompensation, with elevated alanine aminotransferase (ALT), who are positive for serum hepatitis-C-virus (HCV) RNA.
Children and adolescents
Ribavirin Mylan is indicated, in a combination regimen with interferon alfa-2b, for the treatment of children and adolescents three years of age and older, who have all types of chronic hepatitis C except genotype 1, not previously treated, without liver decompensation, and who are positive for serum HCV RNA. When deciding to not to defer treatment until adulthood, it is important to consider that the combination therapy induced a growth inhibition. The reversibility of growth inhibition is uncertain. The decision to treat should be made on a case-by-case basis (see section 4. 4).
* Previously treatment-failure patients:
Adult patients
Ribavirin Mylan is indicated, in combination with interferon alfa-2b, for the treatment of adult patients with chronic hepatitis C who have previously responded (with normalisation of ALT at the end of treatment) to interferon alpha monotherapy but who have subsequently relapsed.
Ribavirin Teva Pharma B. V. is indicated in combination with other medicinal products for the treatment of
chronic hepatitis C (CHC) in adults (see sections 4. 2, 4. 4, and 5. 1).
Ribavirin Teva Pharma B. V. is indicated in combination with other medicinal products for the treatment of
chronic hepatitis C (CHC) for paediatric patients (children 3 years of age and older and adolescents) not
previously treated and without liver decompensation (see sections 4. 2, 4. 4 and 5. 1).
Ribavirin Teva is indicated for the treatment of chronic hepatitis C virus (HCV) infection in adults, children 3 years of age and older and adolescents and must only be used as part of a combination regimen with interferon alfa-2b. Ribavirin monotherapy must not be used.
There is no safety or efficacy information on the use of Ribavirin with other forms of interferon (i. e. not alfa-2b).
* Nave patients:
*:
Adult patients
Ribavirin Teva is indicated, in combination with interferon alfa-2b, for the treatment of adult patients with all types of chronic hepatitis C except genotype 1, not previously treated, without liver decompensation, with elevated alanine aminotransferase (ALT), who are positive for hepatitis C viral ribonucleic acid HCV-RNA.
Paediatric patients (children 3 years of age and older and adolescents)
Ribavirin Teva is indicated, in a combination regimen with interferon alfa2b, for the treatment of children and adolescents 3 years of age and older, who have all types of chronic hepatitis C except genotype 1, not previously treated, without liver decompensation, and who are positive for HCV-RNA.
When deciding not to defer treatment until adulthood, it is important to consider that the combination therapy induced a growth inhibition that may be irreversible in some patients. The reversibility of growth inhibition is uncertain. The decision to treat should be made on a case by case basis.
* Previous treatment failure patients:
Adult patients
Ribavirin Teva is indicated, in combination with interferon alfa-2b, for the treatment of adult patients with chronic hepatitis C who have previously responded (with normalisation of ALT at the end of treatment) to interferon alpha monotherapy but who have subsequently relapsed.
Ribavirin BioPartners is indicated for the treatment of chronic hepatitis-C-virus (HCV) infection in adults, children three years of age and older and adolescents and must only be used as part of a combination regimen with interferon alfa-2b. Ribavirin monotherapy must not be used. There is no safety or efficacy information on the use of ribavirin with other forms of interferon (i. e. not alfa-2b).
* Nave patients:
Adult patients
Ribavirin BioPartners is indicated, in combination with interferon alfa-2b, for the treatment of adult patients with all types of chronic hepatitis C except genotype 1, not previously treated, without liver decompensation, with elevated alanine aminotransferase (ALT), who are positive for hepatitis C viral ribonucleic acid (HCV-RNA) (see section 4. 4)
Children three years of age and older and adolescents
Ribavirin BioPartners is intended for use, in a combination regimen with interferon alfa-2b, for the treatment of children three years of age and older and adolescents, who have all types of chronic hepatitis C except genotype 1, not previously treated, without liver decompensation, and who are positive for HCV-RNA.
When deciding to not to defer treatment until adulthood, it is important to consider that the combination therapy induced a growth inhibition. The reversibility of growth inhibition is uncertain. The decision to treat should be made on a case by case basis (see section 4. 4).
* Previous-treatment-failure patients:
Adult patients
Ribavirin BioPartners is indicated, in combination with interferon alfa-2b, for the treatment of adult patients with chronic hepatitis C who have previously responded (with normalisation of ALT at the end of treatment) to interferon alfa monotherapy but who have subsequently relapsed (see section 5. 1).
Cotronak is indicated for the treatment of chronic hepatitis C and must only be used as part of a combination regimen with peginterferon alfa-2b or interferon alfa-2b. Cotronak monotherapy must not be used.
There is no safety or efficacy information on the use of Cotronak with other forms of interferon (i. e. , not alfa-2b).
Please refer also to the peginterferon alfa-2b or interferon alfa-2b Summary of Product Characteristics (SPC) for prescribing information particular to that product.
Treatment of chronic viral hepatitis C
Ribavirin mediates direct antiviral activity against a number of DNA and RNA viruses by increasing the mutation frequency in the genomes of several RNA viruses. It is a member of the nucleoside antimetabolite drugs that interfere with duplication of the viral genetic material. The drug inhibits the activity of the enzyme RNA dependent RNA polymerase, due to its resemblence to building blocks of the RNA molecules.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J05AP01
J05AB04
J05AP01
J05AB04
J05AB04
J05AB04
J05AP01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AP - Antivirals for treatment of hcv infections
J05AP01 - Ribavirin
Absorption
Ribavirin is reported to be rapidly and extensively absorbed following oral administration. The average time to reach Cmax was 2 hours after oral administration of 1200 mg ribavirin. The oral bioavailability is 64% following a single oral dose administration of 600mg ribavirin.
Route of Elimination
The metabolites of ribavirin are renally excreted. After the oral administration of 600mg radiolabeled ribavirin, approximately 61% of the drug was detected in the urine and 12% was detected in the feces. 17% of administered dose was in unchanged form.
Volume of Distribution
Ribavirin displays a large volume of distribution.
Clearance
The total apparent clearance rate after a single oral dose administration of 1200 mg ribavirin is 26L/h.
Ribavirin is absorbed systemically from the respiratory tract following nasal and oral inhalation. The bioavailability of ribavirin administered via nasal and oral inhalation has not been determined but may depend on the method of drug delivery during nebulization (eg, oxygen hood, face mask, oxygen tent). At a constant flow rate, the amount of drug delivered to the respiratory tract theoretically is directly related to the concentration of nebulized drug solution and the duration of inhalation therapy. In addition, alterations in the method of aerosol delivery can affect the amount of drug reaching the respiratory tract. The fraction of an inhaled dose of ribavirin that is deposited in the respiratory tract during oral and nasal inhalation of a nebulized solution containing 190 ug/L using a small particle aerosol generator has been estimated to average about 70%, but the actual amount deposited depends on several factors including respiratory rate and tidal volume.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 810
Peak plasma ribavirin concentrations generally appear to occur at the end of the inhalation period when the drug is inhaled orally and nasally using a small particle aerosol generator, and increase with increasing duration of the inhalation period. Following nasal and oral inhalation (via face mask) of 0.82 mg/kg/hr for 2.5 hr daily for 3 days in a limited number of pediatric patients, peak plasma ribavirin concentrations averaged 0.19 (range: 0.11-0.388) ug/mL. Peak plasma ribavirin concentrations averaged 0.275 (range: 0.21-0.35) or 1.1 (range: 0.45-2.18) ug/mL in a limited number of patients inhaling 0.82 mg/kg per hour for 5 or 8 hr daily, respectively, for 3 days, and averaged 1.7 (range: 0.38-3.58) ug/mL in a limited number of pediatric patients inhaling 0.82 mg/kg per hour via face mask, mist tent, or respirator for 20 hr daily for 5 days. Highest plasma concentrations for a given dosage of ribavirin appear to be achieved in patients receiving the drug from the aerosol generator via an endotracheal tube. ... Peak plasma ribavirin concentrations achieved with nasal and oral inhalation of usual dosages of the drug are less than concentrations that reportedly reduce respiratory syncytial virus plaque formation by 85-98%.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 810
Concentrations of ribavirin achieved in respiratory tract secretions in patients inhaling the drug nasally and orally are likely to be substantially greater than those achieved in plasma. In a limited number of pediatric patients who received a nasally and orally inhaled ribavirin dose of 0.82 mg/kg per hour for 8 hr daily for 3 days, peak concentrations of the drug in respiratory tract secretions (from endotracheal tube) ranged from 250-1925 ug/mL. In pediatric patients who received 0.82 mg/kg per hour via nasal and oral inhalation for 20 hr daily for 5 days, ribavirin concentrations in respiratory tract secretions (from endotracheal tube) ranged from 313-28,250 ug/mL during therapy, with peak concentrations averaging 3075 (range: 313-7050) ug/mL at the end of therapy. Concentrations of ribavirin achieved in respiratory tract secretions via nasal and oral inhalation are likely to be substantially greater than concentrations necessary to inhibit plaque formation of susceptible strains of respiratory syncytial virus in vitro; however, because respiratory syncytial virus is found within virus infected cells in the respiratory tract, the manufacturer states that intracellular respiratory tract drug concentrations may be more closely related to plasma ribavirin concentrations than to those measured in respiratory tract secretions.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 810
Ribavirin is rapidly absorbed following oral administration, with peak plasma concentrations of the drug occurring within 1-3 hr after multiple doses. However, the absolute bioavailability of ribavirin averages only 64% following oral administration because the drug undergoes first-pass metabolism.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 810
For more Absorption, Distribution and Excretion (Complete) data for RIBAVIRIN (10 total), please visit the HSDB record page.
First and as a step required for activation, ribavirin is phosphorylated intracellularly by adenosine kinase to ribavirin mono-, di-, and triphosphate metabolites. After activation and function, ribavirin undergoes two metabolic pathways where it is reversibly phosphorlyated or degraded via deribosylation and amide hydrolysis to yield a triazole carboxylic acid metabolite. In vitro studies indicate that ribavirin is not a substrate of CYP450 enzymes.
Ribavirin is metabolized principally to deribosylated ribavirin (the 1,2,4-triazole-3-carboxamide), probably in the liver; the antiviral activity of 1,2,4-triazole-3-carboxamide against various RNA and DNA viruses is reportedly similar to ribavirin. The drug is also metabolized to 1,2,4-triazole-3-carboxylic acid. In vitro, ribavirin has been shown to be metabolized to ribavirin-5'-monophosphate, -diphosphate, and -triphosphate, principally by intracellular phosphorylation of the drug via adenosine kinase and other cellular enzymes. It is likely that phosphorylation in vivo is necessary for the antiviral activity of the drug. Ribavirin also undergoes phosphorylation in erythrocytes, principally to ribavirin-5'-triphosphate; approximately 81, 16, and 3% of drug metabolized in erythrocytes is present as ribavirin-5'-triphosphate, -diphosphate, and -monophosphate, respectively. It has been suggested that prolonged distribution of the drug in erythrocytes may result from minimal phosphatase activity in these cells with transit of the drug out of cells dependent on dephosphorylation via phosphatases.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 811
Ribavirin has two pathways of metabolism: (i) a reversible phosphorylation pathway in nucleated cells; and (ii) a degradative pathway involving deribosylation and amide hydrolysis to yield a triazole carboxylic acid metabolite. Ribavirin and its triazole carboxamide and triazole carboxylic acid metabolites are excreted renally.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 3083
The terminal half-life of ribavirin following administration of a single oral dose of 1200 mg is about 120 to 170 hours.
Distribution: Intravenous: Approximately 0.2 hours. Elimination: inhalation: 9.5 hours. Intravenous and oral (single dose): 0.5 to 2 hours. In erythrocytes: 40 days. Terminal: Intravenous and oral: Single dose: 27 to 36 hours. Single oral dose tablet: 120 to 170 hours. Steady state: Approximately 151 hours. Mean :multiple oral dosing, capsule: 298 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2585
Based on limited data, the half-life of ribavirin in respiratory tract secretions following nasal and oral inhalation for 3 days reportedly is approximately 1.4-2.5 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 811
Following nasal and oral inhalation in a limited number of pediatric patients, the plasma half-life of ribavirin averaged about 9.5 (range: 6.5-11) hr. Following oral administration of a single dose of the drug in a limited number of healthy adults, plasma ribavirin concentrations declined in a multiphasic manner, with half-lives averaging 24 hr 10-80 hr after the dose and 48 hr or longer in the terminal phase.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 811
Ribavirin is reported to have several mechanism of actions that lead to inhibition of viral RNA and protein synthesis. After activation by adenosine kinase to ribavirin mono-, di-, and triphosphate metabolites. Ribavirin triphosphate (RTP) is the predominant metabolite which directly inhibits viral mRNA polymerase by binding to the nucleotide binding site of the enzyme. This prevents the binding of the correct nucleotides, leading to a reduction in viral replication or to the production of defective virions. RTP also demonstrates an inhibitory action on viral mRNA guanylyltransferase and mRNA 2-O-methyltransferase of dengue virus. Inhibition of these enzymes disrupts the posttranslational capping of the 5 end of viral mRNA through ribavirin being incorporated at the 5 end in place of guanosine and preventing the cap methylation step. Inhibition of host inosine monophosphate dehydrogenase (IMPDH) and subsequent depletion of GTP pool is proposed to be another mechanism of action of ribavirin. IMPDH catalyzes the rate-limiting step where inosine 5-monophosphate is converted to xanthine monophosphate during guanosine monophosphate (GMP) synthesis. GMP is later converted to guanosine triphoshpate (GTP). Ribavirin monophosphate mimics inosine 5-monophosphate and acts as a competitive inhibitor of IMPDH. Inhibited de novo synthesis of guanine nucleotides and decreased intracellular GTP pools leads to a decline in viral protein synthesis and limit replication of viral genomes. Ribavirin acts as a mutagen in the target virus to cause an 'error catastrophe' due to increased viral mutations. RTP pairs with cytidine triphosphate or uridine triphosphate with equal efficiency and to block HCV RNA elongation. It causes premature termination of nascent HCV RNA and increases mutagenesis by producing defective virions. Ribavirin also exerts an immunomodulatory action of the host to the virus by shifting a Th2 response in favor of a Th1 phenotype. Th2 response and production of type 2 cytokines such as IL-4, IL-5, and IL-10 stimulates the humoral response which enhances immunity toward the virus. Ribavirin enhanced induction of interferon-related genes, including the interferon- receptor, and down-regulation of genes involved in interferon inhibition, apoptosis, and hepatic stellate cell activation in vitro.
The exact mechanism of action of the antiviral activity of ribavirin has not been fully elucidated, but the drug appears to exert its antiviral activity by interfering with RNA and DNA synthesis and subsequently inhibiting protein synthesis and viral replication. The antiviral activity of the drug results principally in an intracellular virustatic effect in cells infected with ribavirin sensitive RNA or DNA viruses; however, specific mechanisms of action of the drug may vary depending on the virus. In virus infected cells in vitro, ribavirin generally exhibits a greater affinity for inhibition of viral DNA and RNA synthesis than cellular (host cell) DNA and RNA synthesis. However, in vesicular stomatitis virus infected cells in vitro, the drug appeared to exhibit a greater affinity for inhibition of cellular than viral RNA synthesis. Inhibition of cellular RNA synthesis usually occurs only at in vitro concentrations higher than those necessary for inhibition of cellular DNA synthesis.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 809
The antiviral activity of ribavirin appears to depend principally on intracellular conversion of the drug to ribavirin-5'-triphosphate and -monophosphate. Ribavirin-5'-diphosphate exhibits minimal antiviral activity compared with the monophosphate or triphosphate. Ribavirin is readily absorbed across the cellular plasma membrane, probably via a nucleoside transport mechanism. The drug is then converted via cellular enzymes to deribosylated ribavirin (the 1,2,4-triazole-3-carboxamide) and phosphorylated to ribavirin-5'-monophosphate, -diphosphate, and -triphosphate. Phosphorylation of ribavirin occurs principally in virus infected cells, but also occurs in uninfected cells. Ribavirin is converted to ribavirin-5'-monophosphate via adenosine kinase; the monophosphate is phosphorylated to the diphosphate and triphosphate via other cellular enzymes, including adenosine kinase. The enzyme deoxyadenosine kinase may also participate in the phosphorylation of ribavirin. Formation of ribavirin-5'-monophosphate appears to be the rate limiting step in the formation of ribavirin-5'-triphosphate. The extent of phosphorylation of ribavirin by both uninfected and virus-infected cells in vitro is directly related to the extracellular (eg, in the culture medium) concentration of the drug. Ribavirin-5'-triphosphate is the principal intracellular form of the drug,with only approximately 4 and 12% of the phosphorylated metabolites present as ribavirin-5'-diphosphate and -monophosphate, respectively. Transit of the drug out of cells appears to occur only after dephosphorylation via phosphatases.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 809
In vitro studies with influenza virus indicate that ribavirin-5'-triphosphate functions as a preferential inhibitor of viral RNA polymerase. Ribavirin-5'-triphosphate competes with adenosine-5'-triphosphate and guanosine-5'-triphosphate for viral RNA polymerase. Inhibition of cellular (host cell) RNA polymerase reportedly is minimal and reversible. In vitro studies with influenza virus have shown that ribavirin-5'-triphosphate also inhibits viral replication by inhibiting guanylyltransferase and methyltransferase, enzymes necessary for the addition of guanosine triphosphate to the 5' terminus ("cap") of viral messenger RNA (mRNA), and by competing with guanosine for incorporation into the 5' terminus of viral mRNA. Although the rate of synthesis of mRNA does not appear to be affected, the efficiency of translation of mRNA inviral replication is decreased by about 80%. Viruses in which the 5' mRNA terminus is naturally absent (eg, poliovirus) are generally not substantially inhibited by ribavirin.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 809
In vitro studies indicate that ribavirin inhibits phosphorylation of thymidine at drug concentrations of 2 umol/L (0.5 ug/mL) and that DNA synthesis is inhibited only at drug concentrations of 200 umol/L (50 ug/mL). Unlike acyclovir, ribavirin appears to be incorporated minimally, if at all, into growing chains of DNA and RNA. In vitro studies with vaccinia virus have shown that the virus DNA fails to coat in the presence of ribavirin, resulting in incomplete viral particles.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 809
At lower dosages, stimulation of antibody formation against some viruses has been reported. The drug has been shown to stimulate T cells (T-lymphocytes) indirectly by inhibiting splenic suppressor cells and to produce a dose dependent inhibition of antigen and mitogen induced proliferation of lymphocytes without affecting cell survival. In human infants, antibody formation against respiratory syncytial virus has decreased during therapy with ribavirin, but the clinical importance of this finding is not known. The drug has been shown to have little, if any, effect on antibody formation against influenza A or B or measles virus in infected patients. Decreases in antibody formation during viral infections may result from decreases in antigenic stimulation secondary to ribavirin-induced inhibition of viral replication or from a direct inhibition of antibody formation by the drug. Ribavirin may indirectly inhibit respiratory syncytial virus specific immunoglobulin E and histamine, which are increased in infants who have wheezing in association with respiratory syncytial virus infection, by decreasing respiratory syncytial virus and attendant antigenic stimulation.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 809
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
83
PharmaCompass offers a list of Ribavirin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ribavirin manufacturer or Ribavirin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ribavirin manufacturer or Ribavirin supplier.
PharmaCompass also assists you with knowing the Ribavirin API Price utilized in the formulation of products. Ribavirin API Price is not always fixed or binding as the Ribavirin Price is obtained through a variety of data sources. The Ribavirin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ribavirin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ribavirin, including repackagers and relabelers. The FDA regulates Ribavirin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ribavirin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ribavirin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ribavirin supplier is an individual or a company that provides Ribavirin active pharmaceutical ingredient (API) or Ribavirin finished formulations upon request. The Ribavirin suppliers may include Ribavirin API manufacturers, exporters, distributors and traders.
click here to find a list of Ribavirin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ribavirin DMF (Drug Master File) is a document detailing the whole manufacturing process of Ribavirin active pharmaceutical ingredient (API) in detail. Different forms of Ribavirin DMFs exist exist since differing nations have different regulations, such as Ribavirin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ribavirin DMF submitted to regulatory agencies in the US is known as a USDMF. Ribavirin USDMF includes data on Ribavirin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ribavirin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ribavirin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Ribavirin Drug Master File in Japan (Ribavirin JDMF) empowers Ribavirin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Ribavirin JDMF during the approval evaluation for pharmaceutical products. At the time of Ribavirin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Ribavirin suppliers with JDMF on PharmaCompass.
A Ribavirin CEP of the European Pharmacopoeia monograph is often referred to as a Ribavirin Certificate of Suitability (COS). The purpose of a Ribavirin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ribavirin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ribavirin to their clients by showing that a Ribavirin CEP has been issued for it. The manufacturer submits a Ribavirin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ribavirin CEP holder for the record. Additionally, the data presented in the Ribavirin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ribavirin DMF.
A Ribavirin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ribavirin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ribavirin suppliers with CEP (COS) on PharmaCompass.
A Ribavirin written confirmation (Ribavirin WC) is an official document issued by a regulatory agency to a Ribavirin manufacturer, verifying that the manufacturing facility of a Ribavirin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ribavirin APIs or Ribavirin finished pharmaceutical products to another nation, regulatory agencies frequently require a Ribavirin WC (written confirmation) as part of the regulatory process.
click here to find a list of Ribavirin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ribavirin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ribavirin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ribavirin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ribavirin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ribavirin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ribavirin suppliers with NDC on PharmaCompass.
Ribavirin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ribavirin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ribavirin GMP manufacturer or Ribavirin GMP API supplier for your needs.
A Ribavirin CoA (Certificate of Analysis) is a formal document that attests to Ribavirin's compliance with Ribavirin specifications and serves as a tool for batch-level quality control.
Ribavirin CoA mostly includes findings from lab analyses of a specific batch. For each Ribavirin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ribavirin may be tested according to a variety of international standards, such as European Pharmacopoeia (Ribavirin EP), Ribavirin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ribavirin USP).
