
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
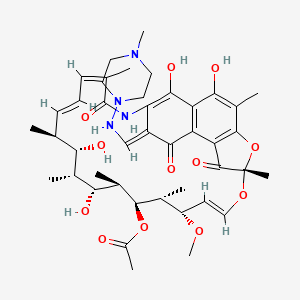

1. Rifomycin S
2. Rifampicin S
| Molecular Weight | 822.9 g/mol |
|---|---|
| Molecular Formula | C43H58N4O12 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 5 |
| Exact Mass | 822.40512330 g/mol |
| Monoisotopic Mass | 822.40512330 g/mol |
| Topological Polar Surface Area | 217 A^2 |
| Heavy Atom Count | 59 |
| Formal Charge | 0 |
| Complexity | 1750 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Rifadin |
| PubMed Health | Rifampin (Injection) |
| Drug Classes | Antitubercular |
| Drug Label | RIFADIN (rifampin capsules USP) for oral administration contain 150 mg or 300 mg rifampin per capsule. The 150 mg and 300 mg capsules also contain, as inactive ingredients: corn starch, D&C Red No. 28, FD&C Blue No. 1, FD&C Red No. 40, gelatin |
| Active Ingredient | Rifampin |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 150mg; 300mg; 600mg/vial |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 6 | |
|---|---|
| Drug Name | Rifampin |
| Active Ingredient | Rifampin |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 150mg; 300mg; 600mg/vial |
| Market Status | Prescription |
| Company | Bedford; Lupin Pharms; Sandoz; Emcure Pharms; Fresenius Kabi Usa; Versapharm; Lannett; Agila Speclts |
| 3 of 6 | |
|---|---|
| Drug Name | Rimactane |
| PubMed Health | Rifampin |
| Drug Classes | Antitubercular |
| Drug Label | Rifampin is a semisynthetic antibiotic derivative of rifamycin SV. Rifampin is a red-brown crystalline powder very slightly soluble in water at neutral pH, freely soluble in chloroform, soluble in ethyl acetate and in methanol. Its molecular weight i... |
| Active Ingredient | Rifampin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Prosam Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Rifadin |
| PubMed Health | Rifampin (Injection) |
| Drug Classes | Antitubercular |
| Drug Label | RIFADIN (rifampin capsules USP) for oral administration contain 150 mg or 300 mg rifampin per capsule. The 150 mg and 300 mg capsules also contain, as inactive ingredients: corn starch, D&C Red No. 28, FD&C Blue No. 1, FD&C Red No. 40, gelatin |
| Active Ingredient | Rifampin |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 150mg; 300mg; 600mg/vial |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 5 of 6 | |
|---|---|
| Drug Name | Rifampin |
| Active Ingredient | Rifampin |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 150mg; 300mg; 600mg/vial |
| Market Status | Prescription |
| Company | Bedford; Lupin Pharms; Sandoz; Emcure Pharms; Fresenius Kabi Usa; Versapharm; Lannett; Agila Speclts |
| 6 of 6 | |
|---|---|
| Drug Name | Rimactane |
| PubMed Health | Rifampin |
| Drug Classes | Antitubercular |
| Drug Label | Rifampin is a semisynthetic antibiotic derivative of rifamycin SV. Rifampin is a red-brown crystalline powder very slightly soluble in water at neutral pH, freely soluble in chloroform, soluble in ethyl acetate and in methanol. Its molecular weight i... |
| Active Ingredient | Rifampin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Prosam Labs |

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : RIFADIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG
Packaging :
Approval Date : 1982-01-01
Application Number : 50420
Regulatory Info : DISCN
Registration Country : USA
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : RX
Registration Country : USA
Brand Name : RIFADIN
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 600MG/VIAL
Packaging :
Approval Date : 1989-05-25
Application Number : 50627
Regulatory Info : RX
Registration Country : USA
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : DISCN
Registration Country : USA
ISONIAZID; PYRAZINAMIDE; RIFAMPIN
Brand Name : RIFATER
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG;300MG;120MG
Packaging :
Approval Date : 1994-05-31
Application Number : 50705
Regulatory Info : DISCN
Registration Country : USA
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : RIFADIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : 150MG
Packaging :
Approval Date : 1982-01-01
Application Number : 62303
Regulatory Info : DISCN
Registration Country : USA
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Sweden
Brand Name : Rifadin
Dosage Form : PULVER OCH VÄTSKA TILL INFUSIONSVÄTSKA
Dosage Strength : 600 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Isoniazid; Pyrazinamide; Rifampin
Brand Name : Rifater
Dosage Form : Isoniazid+Pyrazinamide+Rifampicin 50+300+120Mg 40 Combined Oral Use
Dosage Strength : 40 cpr riv 50 mg + 300mg + 120 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Brand Name : Rifadin
Dosage Form : Rifampicin 300Mg 8 Joined' Oral Use
Dosage Strength : 8 cps 300 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Brand Name : Rifadin
Dosage Form : Rifampicin 600Mg 1 Unit Parenteral Use
Dosage Strength : 1 ampoule EV 600 mg + 1 ampoule solv 10 ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Brand Name : Rifinah
Dosage Form : Rifampicin+Isoniazid+150300 Mg 24 Units Of Oral Use
Dosage Strength : 24 cpr riv 300 mg + 150 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Rifampicinum; Isoniazidum; Pyrazinamidum
Brand Name : Rifater
Dosage Form : Drag
Dosage Strength : 50mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Regulatory Info : Dossier Availability: Q4 2026
Registration Country : Poland
Brand Name :
Dosage Form : Hard Capsule
Dosage Strength : 150MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Dossier Availability: Q4 2026
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Packaging :
Regulatory Info : Dossier Availability: Q4 2026
Dosage : Hard Capsule
Dosage Strength : 150MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Regulatory Info : Dossier Availability: Q4 2026
Registration Country : Poland
Brand Name :
Dosage Form : Hard Capsule
Dosage Strength : 300MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Dossier Availability: Q4 2026
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Packaging :
Regulatory Info : Dossier Availability: Q4 2026
Dosage : Hard Capsule
Dosage Strength : 300MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Regulatory Info : Dossier Availability: Q4 2026
Registration Country : Poland
Brand Name :
Dosage Form : Hard Capsule
Dosage Strength : 150MG; 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Dossier Availability: Q4 2026
Registration Country : Poland
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Packaging :
Regulatory Info : Dossier Availability: Q4 2026
Dosage : Hard Capsule
Dosage Strength : 150MG; 100MG
Brand Name :
Approval Date :
Application Number :
Registration Country : Poland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Romania
Brand Name : SINERDOL®
Dosage Form : capsules
Dosage Strength : 150MG
Packaging : Box x 20 capsules Box x 1000 capsules
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Romania

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : Box x 20 capsules Box x 1000 capsules
Regulatory Info :
Dosage : capsules
Dosage Strength : 150MG
Brand Name : SINERDOL®
Approval Date :
Application Number :
Registration Country : Romania

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Romania
Brand Name : SINERDOL®
Dosage Form : capsules
Dosage Strength : 300MG
Packaging : Box x 10 capsules Box x 1000 capsules
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Romania

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : Box x 10 capsules Box x 1000 capsules
Regulatory Info :
Dosage : capsules
Dosage Strength : 300MG
Brand Name : SINERDOL®
Approval Date :
Application Number :
Registration Country : Romania

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Pellet
Dosage Strength : 60%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Pellet
Dosage Strength : 60%
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name :
Dosage Form : Injection
Dosage Strength : 600MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 600MG
Brand Name :
Approval Date :
Application Number :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Hungary
Brand Name : Rifamed
Dosage Form : Film Coated Tablet
Dosage Strength : 300MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 300MG
Brand Name : Rifamed
Approval Date :
Application Number :
Registration Country : Hungary

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Hungary
Brand Name : Rifamed
Dosage Form : Film Coated Tablet
Dosage Strength : 150MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Film Coated Tablet
Dosage Strength : 150MG
Brand Name : Rifamed
Approval Date :
Application Number :
Registration Country : Hungary

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : URIFA
Dosage Form : CAPSULE
Dosage Strength : 150MG
Packaging : Blister of 10s
Approval Date :
Application Number : 90034
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : Blister of 10s
Regulatory Info : Generic
Dosage : CAPSULE
Dosage Strength : 150MG
Brand Name : URIFA
Approval Date :
Application Number : 90034
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?