
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 4-deoxy-4'-methylpyrido(1',2'-1,2)imidazo(5,4c)rifamycin
2. L 105
3. L-105
4. L105
5. Redactiv
6. Xifaxan
1. Rifaxidin
2. Rifacol
3. 80621-81-4
4. Rifamycin L 105
5. Xifaxan
6. Rifamycin L 105sv
7. Fatroximin
8. Rifaximine
9. Normix
10. Rifaximina
11. Xifaxsan
12. L-105
13. Rifamixin
14. Rifaximine [french]
15. Rifaximinum [latin]
16. Rifaximina [spanish]
17. Ritacol
18. Chebi:75246
19. 4-deoxy-4'-methylpyrido(1',2'-1,2)imidazo(5,4-c)rifamycin Sv
20. L36o5t016n
21. Rifaximin (xifaxan)
22. Nsc-758957
23. Rifaximinum
24. Brn 3584528
25. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-1,15-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)furo[2'',3'':7',8']naphtho[1',2':4,5]imidazo[1,2-a]pyridin-25-yl Acetate
26. C43h51n3o11
27. Rifaxin
28. Redactiv
29. Ido[1,2-a]benzimidazol-25-yl Acetate
30. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca[1,11,13]trienimino)benzofuro[4,5-e]pyrido[1,2-a]benzimidazole-1,15(2h)-dione
31. L 105sv
32. L 105 (ansamacrolide Antibiotic)
33. L 105
34. Rifaximinun
35. Flonorm
36. Lumenax
37. Spiraxin
38. Lormyx
39. Rifaximin [usan:inn:ban]
40. Unii-l36o5t016n
41. 5-yl Acetate
42. Ncgc00095842-01
43. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione
44. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-1,15-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)[1]benzofuro[4,5-e]pyr
45. Xifaxan (tn)
46. Mfcd00864973
47. Rifaximin [inn]
48. Rifaximin [jan]
49. Rifaximin [mi]
50. Rifaximin [usan]
51. Rifaximin [vandf]
52. Rifaximin [mart.]
53. Alpha-0817185
54. Rifaximin [who-dd]
55. Chembl1617
56. Dsstox_cid_25998
57. Dsstox_rid_81280
58. Dsstox_gsid_45998
59. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26s,27s,28e)-5,6,21,23,25 Pentahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione, 25-acetate
60. Rifaximin (jan/usan/inn)
61. Schembl124066
62. Rifaximin [ep Impurity]
63. Rifaximin [orange Book]
64. Dtxsid7045998
65. Rifaximin [ep Monograph]
66. Gtpl12012
67. Hms3715b19
68. 88747-56-2
69. Tox21_111529
70. Bdbm50347620
71. S1790
72. Akos015963053
73. Zinc169621200
74. Ccg-221129
75. Db01220
76. Nsc 758957
77. Rifaximin 100 Microg/ml In Acetonitrile
78. 2,7-(epoxy(1,11,13)pentadecatrienoimino)furo(2'',3'':7',8')naphth(1',2':4,5)imidazo(1,2-a)pyridine-1,15(2h)-dione, 25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-, ( 2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-
79. 2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione, 25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-, (2s-(2r*,16z,18e,20r*,21r*,22s*,23s*,24s*,25r*,26s*,27r*,28e))-
80. Ac-19112
81. Cas-80621-81-4
82. L/105
83. D02554
84. Ab01209738-01
85. Ab01209738-03
86. Ab01209738_04
87. Rifaximin, Antibiotic For Culture Media Use Only
88. 621r814
89. Q416073
90. Q-201671
91. (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-1,1
92. (7s,11s,12r,13s,14r,15r,16r,17s,18s)-2,15,17,36-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22,30-octamethyl-6,23-dioxo-8,37-dioxa-24,27,33-triazahexacyclo[23.10.1.1^{4,7}.0^{5,35}.0^{26,34}.0^{27,32}]heptatriaconta-1,3,5(35),9,19,21,25(36),26(34),28,30,32-undecaen-13-yl Acetate
93. [(7s,9e,11s,12r,13s,14r,15r,16r,17s,18s,19e,21z)-2,15,17,36-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22,30-octamethyl-6,23-dioxo-8,37-dioxa-24,27,33-triazahexacyclo[23.10.1.14,7.05,35.026,34.027,32]heptatriaconta-1(35),2,4,9,19,21,25(36),26(34),28,30,32-undecaen-13-yl] Acetate
94. 2,7-(epoxypentadeca(1,11,13)trienimino)benzofuro(4,5-e)pyrido(1,2-a)benzimidazole-1,15(2h)-dione, 25-(acetyloxy)-5,6,21,23-tetrahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-, (2s,16z,18e,20s,21s,22r,23r,24r,25s,26r,27s,28e)-
95. 5-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)furo[2'',3'':7',8']naphtho[1',2':4,5]imidazo[1,2-a]pyridin-2
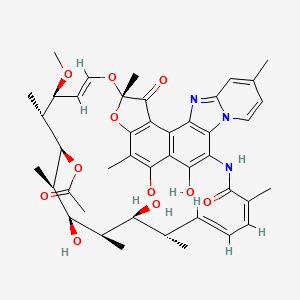
| Molecular Weight | 785.9 g/mol |
|---|---|
| Molecular Formula | C43H51N3O11 |
| XLogP3 | 6.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 3 |
| Exact Mass | 785.35235945 g/mol |
| Monoisotopic Mass | 785.35235945 g/mol |
| Topological Polar Surface Area | 198 Ų |
| Heavy Atom Count | 57 |
| Formal Charge | 0 |
| Complexity | 1590 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Xifaxan |
| PubMed Health | Rifaximin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2 ,16 ,18 ,20 ,21 ,22 ,23 ,24 ,25 ,26 ,27... |
| Active Ingredient | Rifaximin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 550mg |
| Market Status | Prescription |
| Company | Salix Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Xifaxan |
| PubMed Health | Rifaximin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2 ,16 ,18 ,20 ,21 ,22 ,23 ,24 ,25 ,26 ,27... |
| Active Ingredient | Rifaximin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 550mg |
| Market Status | Prescription |
| Company | Salix Pharms |
Rifaximin has multiple indications by the FDA: for the treatment of patients (12 years of age) with traveller's diarrhea caused by noninvasive strains of Escherichia coli; for the reduction of overt hepatic encephalopathy recurrence in patients 18 years of age; and in May 2015 it was approved for irritable bowel syndrome with diarrhea (IBS-D) treatment in adult men and women.
FDA Label
Rifaximin is a structural analog of rifampin and a non-systemic, gastrointestinal site-specific antibiotic. This non-systemic property of the drug is due to the addition of a pyridoimidazole ring, which renders it non-absorbable. Rifaximin acts by inhibiting bacterial ribonucleic acid (RNA) synthesis and contributes to restore intestinal microflora imbalance. Other studies have also shown rifaximin to be an pregnane X receptor (PXR) activator. As PXR is responsible for inhibiting the proinflammatory transcription factor NF-kappa B (NF-B) and is inhibited in inflammatory bowel disease (IBD), rifaximin was proven to be effective for the treatment of IBS-D.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
A07AA11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AA - Antibiotics
A07AA11 - Rifaximin
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX11 - Rifaximin
Absorption
Low absorption in both the fasting state and when administered within 30 minutes of a high-fat breakfast.
Route of Elimination
In a mass balance study, after administration of 400 mg 14C-rifaximin orally to healthy volunteers, of the 96.94% total recovery, 96.62% of the administered radioactivity was recovered in feces almost exclusively as the unchanged drug and 0.32% was recovered in urine mostly as metabolites with 0.03% as the unchanged drug.Rifaximin accounted for 18% of radioactivity in plasma. This suggests that the absorbed rifaximin undergoes metabolism with minimal renal excretion of the unchanged drug
In vitro drug interactions studies have shown that rifaximin, at concentrations ranging from 2 to 200 ng/mL, did not inhibit human hepatic cytochrome P450 isoenzymes: 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and 3A4. In an in vitro hepa-tocyte induction model, rifaximin was shown to induce cytochrome P450 3A4 (CYP3A4), an isoenzyme which rifampin is known to induce.
Approximately 6 hours.
Rifaximin acts by inhibiting RNA synthesis in susceptible bacteria by binding to the beta-subunit of bacterial deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase enzyme. This binding blocks translocation, which stops transcription.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17150
Submission : 2004-02-10
Status : Active
Type : II

Registration Number : 226MF10147
Registrant's Address : Via Angelo Titi, 22/26, Zona ex punto franco, 72100 Brindisi, Italy
Initial Date of Registration : 2014-09-01
Latest Date of Registration :

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2022-05-04
Registration Number : 20220504-209-J-1285
Manufacturer Name : EUROAPI ITALY SRL
Manufacturer Address : Via Angelo TiTi, 22/26 - 72100 Brindisi (BR)

| Available Reg Filing : ASMF |

 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36125
Submission : 2021-08-29
Status : Active
Type : II

Certificate Number : CEP 2012-146 - Rev 02
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 2362
Status : Valid

NDC Package Code : 62207-020
Start Marketing Date : 2023-08-30
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF |
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Certificate Number : CEP 2012-146 - Rev 02
Status : Valid
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 2362
 Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
 Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
Certificate Number : CEP 2022-368 - Rev 00
Status : Valid
Issue Date : 2024-09-02
Type : Chemical
Substance Number : 2362
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2024-415 - Rev 00
Status : Valid
Issue Date : 2025-07-11
Type : Chemical
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2020-435 - Rev 00
Status : Valid
Issue Date : 2024-02-12
Type : Chemical
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2010-085 - Rev 01
Status : Valid
Issue Date : 2018-10-12
Type : Chemical
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2020-175 - Rev 00
Status : Valid
Issue Date : 2022-12-07
Type : Chemical and TSE
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2016-016 - Rev 01
Status : Valid
Issue Date : 2024-02-28
Type : Chemical
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2019-125 - Rev 04
Status : Valid
Issue Date : 2025-07-25
Type : Chemical
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2024-520 - Rev 00
Status : Valid
Issue Date : 2025-02-06
Type : Chemical
Substance Number : 2362

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2025-071 - Rev 00
Status : Valid
Issue Date : 2025-07-25
Type : Chemical
Substance Number : 2362

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
82
PharmaCompass offers a list of Rifaximin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rifaximin manufacturer or Rifaximin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rifaximin manufacturer or Rifaximin supplier.
PharmaCompass also assists you with knowing the Rifaximin API Price utilized in the formulation of products. Rifaximin API Price is not always fixed or binding as the Rifaximin Price is obtained through a variety of data sources. The Rifaximin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Rifaximin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Rifaximin, including repackagers and relabelers. The FDA regulates Rifaximin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Rifaximin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Rifaximin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Rifaximin supplier is an individual or a company that provides Rifaximin active pharmaceutical ingredient (API) or Rifaximin finished formulations upon request. The Rifaximin suppliers may include Rifaximin API manufacturers, exporters, distributors and traders.
click here to find a list of Rifaximin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Rifaximin DMF (Drug Master File) is a document detailing the whole manufacturing process of Rifaximin active pharmaceutical ingredient (API) in detail. Different forms of Rifaximin DMFs exist exist since differing nations have different regulations, such as Rifaximin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Rifaximin DMF submitted to regulatory agencies in the US is known as a USDMF. Rifaximin USDMF includes data on Rifaximin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Rifaximin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Rifaximin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Rifaximin Drug Master File in Japan (Rifaximin JDMF) empowers Rifaximin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Rifaximin JDMF during the approval evaluation for pharmaceutical products. At the time of Rifaximin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Rifaximin suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Rifaximin Drug Master File in Korea (Rifaximin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Rifaximin. The MFDS reviews the Rifaximin KDMF as part of the drug registration process and uses the information provided in the Rifaximin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Rifaximin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Rifaximin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Rifaximin suppliers with KDMF on PharmaCompass.
A Rifaximin CEP of the European Pharmacopoeia monograph is often referred to as a Rifaximin Certificate of Suitability (COS). The purpose of a Rifaximin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Rifaximin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Rifaximin to their clients by showing that a Rifaximin CEP has been issued for it. The manufacturer submits a Rifaximin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Rifaximin CEP holder for the record. Additionally, the data presented in the Rifaximin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Rifaximin DMF.
A Rifaximin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Rifaximin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Rifaximin suppliers with CEP (COS) on PharmaCompass.
A Rifaximin written confirmation (Rifaximin WC) is an official document issued by a regulatory agency to a Rifaximin manufacturer, verifying that the manufacturing facility of a Rifaximin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Rifaximin APIs or Rifaximin finished pharmaceutical products to another nation, regulatory agencies frequently require a Rifaximin WC (written confirmation) as part of the regulatory process.
click here to find a list of Rifaximin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Rifaximin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Rifaximin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Rifaximin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Rifaximin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Rifaximin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Rifaximin suppliers with NDC on PharmaCompass.
Rifaximin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Rifaximin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Rifaximin GMP manufacturer or Rifaximin GMP API supplier for your needs.
A Rifaximin CoA (Certificate of Analysis) is a formal document that attests to Rifaximin's compliance with Rifaximin specifications and serves as a tool for batch-level quality control.
Rifaximin CoA mostly includes findings from lab analyses of a specific batch. For each Rifaximin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Rifaximin may be tested according to a variety of international standards, such as European Pharmacopoeia (Rifaximin EP), Rifaximin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Rifaximin USP).

