
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
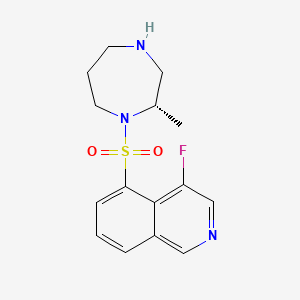

1. Glanatec
2. K-115
3. K115 Compound
1. 223645-67-8
2. K-115 Free Base
3. K115 Free Base
4. Ripasudil [inn]
5. Ripasudil Free Base
6. K-115 (free Base)
7. Chembl3426621
8. 4-fluoro-5-[[(2s)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline
9. 11978226xx
10. (s)-4-fluoro-5-((2-methyl-1,4-diazepan-1-yl)sulfonyl)isoquinoline
11. 1h-1,4-diazepine, 1-((4-fluoro-5-isoquinolinyl)sulfonyl)hexahydro-2-methyl-, (2s)-
12. Isoquinoline, 4-fluoro-5-(((2s)-hexahydro-2-methyl-1h-1,4-diazepin-1-yl)sulfonyl)-
13. Unii-11978226xx
14. K 115 Free Base
15. Ripasudil [mi]
16. Ripasudil [who-dd]
17. Schembl31542
18. Gtpl10423
19. Chebi:136046
20. Dtxsid001025609
21. Bcp11083
22. Ex-a3647
23. Zinc3940873
24. Bdbm50087135
25. Hy-15685a
26. Mfcd28291829
27. Nsc800869
28. Cs-3402
29. Db13165
30. Nsc-800869
31. Ncgc00496843-01
32. Ac-36873
33. As-35170
34. K-115 (ripasudil Hydrochloride Dihydrate)
35. Q21098890
36. (s)-(-)-1-(4-fluoro-5-isoquinolinesulfonyl)-2-methyl-1,4-homopiperazine
37. 4-fluoro-5-[[(2s)-2beta-methylhexahydro-1h-1,4-diazepine-1-yl]sulfonyl]isoquinoline
| Molecular Weight | 323.4 g/mol |
|---|---|
| Molecular Formula | C15H18FN3O2S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 323.11037616 g/mol |
| Monoisotopic Mass | 323.11037616 g/mol |
| Topological Polar Surface Area | 70.7 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 482 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ripasudil has been proven to be effective in the twice daily treatment of glaucoma and ocular hypertension. It is currently in studies to be approved for both diabetic retinopathy and diabetic macular oedema.
Treatment of corneal dystrophy
Ripasudil has high intraocular permeability and works by decreasing intraocular pressure (IOP) in a dose-dependent manner and increasing flow facility. The maximum reduction of IOP occurs after 1 to 2 hours.
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EX - Other antiglaucoma preparations
S01EX07 - Ripasudil
Route of Elimination
Riapsudil is cleared by the kidneys at a rate of 7.112L/h.
Clearance
Ripasudil has a renal clearance of 7.112 L/h.
The half life of Ripasudil is 0.455 hrs.
Ripasudil is a highly selective and potent Rho-associated coiled/coil-containing kinase protein (ROCK) inhibitor. Rho-kinase (ROCK) is an effector protein of Rho which binds with Rho to form a Rho/Rho-kinase complex. This complex then regulates many physiological functions including smooth muscle contractions, chemotaxis, neural growth and gene expression. ROCK comes in 2 isoforms: ROCK-1 and ROCK-2 and these two isoforms are distributed widely in our tissues including ocular tissues such as the iris, retina, trabecular meshwork and ciliary muscles. Atypical regulation of ROCK levels is involved in the pathogenesis of diseases such as glaucoma, ocular hypertension, cataracts and other retinal disorders. Ripasudil acts as very highly selective and potent inhibitor with an IC50 of Ripasudil with ROCK-1 of 0.051 umol/L and with ROCK-2 of 0.019 umol/L. ROCK inhibitors have efficacy in reducing IOP by acting on the trabecular meshwork in the eye directly to increase conventional outflow through the Schlemms canal. Ripasudil will inhibit ROCK and induce cytoskeletal changes including the retraction and rounding of cell bodies and cause disruption of actin bundles in this trabecular meshwork. This can reduce the compaction of trabecular meshwork tissue and eventually result in increased aqueous outflow in the eye and reduced resistance to fluid flow. Thus, Ripasudil is effective by inducing cytoskeletal changes which are depending on ROCK inhibition. Ripasudil decreases IOP by increasing outflow facility along with modulating the behavior of trabecular meshwork cells and Schlemms canal endothelial (SCE) cell permeability along with a disruption of the tight junction. When Ripasudil is used in combination with prostaglandin analogues it results in increased uveoscleral outflow and when used in combination with beta blockers it results in reduced aqueous production.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
77
PharmaCompass offers a list of Ripasudil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ripasudil manufacturer or Ripasudil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ripasudil manufacturer or Ripasudil supplier.
PharmaCompass also assists you with knowing the Ripasudil API Price utilized in the formulation of products. Ripasudil API Price is not always fixed or binding as the Ripasudil Price is obtained through a variety of data sources. The Ripasudil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ripasudil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ripasudil, including repackagers and relabelers. The FDA regulates Ripasudil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ripasudil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ripasudil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ripasudil supplier is an individual or a company that provides Ripasudil active pharmaceutical ingredient (API) or Ripasudil finished formulations upon request. The Ripasudil suppliers may include Ripasudil API manufacturers, exporters, distributors and traders.
click here to find a list of Ripasudil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ripasudil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ripasudil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ripasudil GMP manufacturer or Ripasudil GMP API supplier for your needs.
A Ripasudil CoA (Certificate of Analysis) is a formal document that attests to Ripasudil's compliance with Ripasudil specifications and serves as a tool for batch-level quality control.
Ripasudil CoA mostly includes findings from lab analyses of a specific batch. For each Ripasudil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ripasudil may be tested according to a variety of international standards, such as European Pharmacopoeia (Ripasudil EP), Ripasudil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ripasudil USP).
