
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
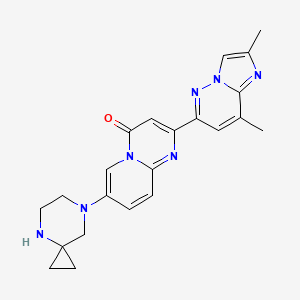

1. 7-(4,7-diazaspiro(2.5)oct-7-yl)-2-(2,8-dimethylimidazo(1,2-b)pyridazin-6-yl)-4h-pyrido(1,2-a)pyrimidin-4-one
1. 1825352-65-5
2. Rg7916
3. Evrysdi
4. Ro7034067
5. Risdiplam [inn]
6. Risdiplam [usan]
7. Rg-7916
8. 76rs4s2et1
9. Ro-7034067
10. 7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)pyrido[1,2-a]pyrimidin-4-one
11. 2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)-7-(4,7-diazaspiro[2.5]octan-7-yl)-4h-pyrido[1,2-a]pyrimidin-4-one
12. 4h-pyrido(1,2-a)pyrimidin-4-one, 7-(4,7-diazaspiro(2.5)oct-7-yl)-2-(2,8-dimethylimidazo(1,2-b)pyridazin-6-yl)-
13. Evrysdi (tn)
14. Risdiplam; Rg7916
15. Risdiplam [jan]
16. Risdiplam [mi]
17. Risdiplam [usan:inn]
18. Risdiplam [who-dd]
19. Unii-76rs4s2et1
20. Risdiplam (jan/usan/inn)
21. Risdiplam [orange Book]
22. Chembl4297528
23. Schembl17260852
24. Gtpl11170
25. Dtxsid701109185
26. Amy23728
27. Ex-a2074
28. Mfcd31657372
29. Who 10614
30. Compound 1 [pmid: 30044619]
31. Db15305
32. Ac-36304
33. Br166842
34. Rg7916;ro7034067
35. Hy-109101
36. Cs-0039501
37. D11406
38. F53623
39. Q48969152
40. (7-(4,7-diazaspiro(2.5)octan-7-yl)-2-(2,8-dimethylimidazo(1,2-b)pyridazin-6-yl)pyrido(1,2-a)pyrimidin-4-one
41. 7-(4,7-diazaspiro(2.5)oct-7-yl)-2-(2,8-dimethylimidazo(1,2-b)pyridazin-6-yl)-4h-pyrido(1,2-a)pyrimidin-4-one
| Molecular Weight | 401.5 g/mol |
|---|---|
| Molecular Formula | C22H23N7O |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 401.19640838 g/mol |
| Monoisotopic Mass | 401.19640838 g/mol |
| Topological Polar Surface Area | 78.1 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 886 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Risdiplam is indicated for the treatment of spinal muscular atrophy (SMA) in patients 2 months of age and older.
Evrysdi is indicated for the treatment of 5q spinal muscular atrophy (SMA) in patients 2 months of age and older, with a clinical diagnosis of SMA Type 1, Type 2 or Type 3 or with one to four SMN2 copies.
Risdiplam helps to alleviate symptoms of spinal muscular atrophy by stimulating the production of a critical protein in which these patients are deficient. Early trials with risdiplam demonstrated up to a 2-fold increase in SMN protein concentration in SMA patients after 12 weeks of therapy.
Neuromuscular Agents
Drugs used for their actions on skeletal muscle. Included are agents that act directly on skeletal muscle, those that alter neuromuscular transmission (NEUROMUSCULAR BLOCKING AGENTS), and drugs that act centrally as skeletal muscle relaxants (MUSCLE RELAXANTS, CENTRAL). Drugs used in the treatment of movement disorders are ANTI-DYSKINESIA AGENTS. (See all compounds classified as Neuromuscular Agents.)
M09AX10
M - Musculo-skeletal system
M09 - Other drugs for disorders of the musculo-skeletal system
M09A - Other drugs for disorders of the musculo-skeletal system
M09AX - Other drugs for disorders of the musculo-skeletal system
M09AX10 - Risdiplam
Absorption
The Tmax following oral administration is approximately 1-4 hours. Following once-daily administration with a morning meal (or after breastfeeding), risdiplam reaches steady-state in approximately 7-14 days. The pharmacokinetics of risdiplam were found to be approximately linear between all studied dosages in patients with SMA.
Route of Elimination
Following the oral administration of 18mg risdiplam, approximately 53% of the dose was excreted in the feces and 28% was excreted in the urine. Unchanged parent drug comprised 14% of the dose excreted in feces and 8% of the dose excreted in urine.
Volume of Distribution
Following oral administration, risdiplam distributes well into the central nervous system and peripheral tissues. The apparent volume of distribution at steady-state is 6.3 L/kg.
Clearance
For a 14.9kg patient, the apparent clearance of risdiplam is 6.3 L/kg.
The metabolism of risdiplam is mediated primarily by flavin monooxygenases 1 and 3 (FMO1 and FMO3), with some involvement of CYP1A1, CYP2J2, CYP3A4, and CYP3A7. Parent drug comprises approximately 83% of circulating drug material. A pharmacologically-inactive metabolite, M1, has been identified as the major circulating metabolite - this M1 metabolite has been observed _in vitro_ to inhibit MATE1 and MATE2-K transporters, similar to the parent drug.
The terminal elimination half-life of risdiplam is approximately 50 hours in healthy adults.
Spinal muscular atrophy (SMA) is a severe and progressive congenital neuromuscular disease resulting from mutations in the survival of motor neuron 1 (_SMN1_) gene responsible for making SMN proteins. Clinical features of SMA include degeneration of motor neurons in the spinal cord which ultimately leads to muscular atrophy and, in some cases, loss of physical strength. SMN proteins are expressed ubiquitously throughout the body and are thought to hold diverse intracellular roles in DNA repair, cell signaling, endocytosis, and autophagy. A secondary _SMN_ gene (_SMN2_) can also produce SMN proteins, but a small nucleotide substitution in its sequence results in the exclusion of exon 7 during splicing in approximately 85% of the transcripts - this means that only ~15% of the SMN proteins produced by _SMN2_ are functional, which is insufficient to compensate for the deficits caused by _SMN1_ mutations. Emerging evidence suggests that many cells and tissues are selectively vulnerable to reduced SMN concentrations, making this protein a desirable target in the treatment of SMA. Risdiplam is an mRNA splicing modifier for _SMN2_ that increases the inclusion of exon 7 during splicing, which ultimately increases the amount of functional SMN protein produced by _SMN2_. It does so by binding to two sites in _SMN2_ pre-mRNA: the 5' splice site (5'ss) of intron 7 and the exonic splicing enhancer 2 (ESE2) of exon 7.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
59
PharmaCompass offers a list of Risdiplam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Risdiplam manufacturer or Risdiplam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Risdiplam manufacturer or Risdiplam supplier.
PharmaCompass also assists you with knowing the Risdiplam API Price utilized in the formulation of products. Risdiplam API Price is not always fixed or binding as the Risdiplam Price is obtained through a variety of data sources. The Risdiplam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Risdiplam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Risdiplam, including repackagers and relabelers. The FDA regulates Risdiplam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Risdiplam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Risdiplam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Risdiplam supplier is an individual or a company that provides Risdiplam active pharmaceutical ingredient (API) or Risdiplam finished formulations upon request. The Risdiplam suppliers may include Risdiplam API manufacturers, exporters, distributors and traders.
click here to find a list of Risdiplam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Risdiplam DMF (Drug Master File) is a document detailing the whole manufacturing process of Risdiplam active pharmaceutical ingredient (API) in detail. Different forms of Risdiplam DMFs exist exist since differing nations have different regulations, such as Risdiplam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Risdiplam DMF submitted to regulatory agencies in the US is known as a USDMF. Risdiplam USDMF includes data on Risdiplam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Risdiplam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Risdiplam suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Risdiplam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Risdiplam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Risdiplam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Risdiplam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Risdiplam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Risdiplam suppliers with NDC on PharmaCompass.
Risdiplam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Risdiplam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Risdiplam GMP manufacturer or Risdiplam GMP API supplier for your needs.
A Risdiplam CoA (Certificate of Analysis) is a formal document that attests to Risdiplam's compliance with Risdiplam specifications and serves as a tool for batch-level quality control.
Risdiplam CoA mostly includes findings from lab analyses of a specific batch. For each Risdiplam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Risdiplam may be tested according to a variety of international standards, such as European Pharmacopoeia (Risdiplam EP), Risdiplam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Risdiplam USP).

