
Synopsis
Synopsis
0
VMF
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
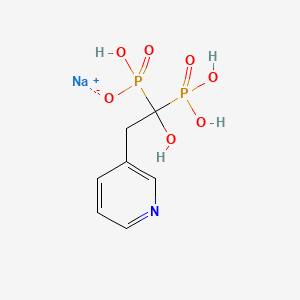

1. 1-hydroxy-2-(3-pyridyl)ethylidene Diphosphonate
2. 2-(3-pyridinyl)-1-hydroxyethylidene-bisphosphonate
3. 2-(3-pyridinyl)-1-hydroxyethylidenebisphosphonate
4. Actonel
5. Atelvia
6. Bisphosphonate Risedronate Sodium
7. Risedronate
8. Risedronate Sodium, Bisphosphonate
9. Risedronic Acid
10. Risedronic Acid, Monosodium Salt
11. Sodium, Bisphosphonate Risedronate
1. Sodium Risedronate
2. 115436-72-1
3. Actonel
4. Atelvia
5. Risedronate (sodium)
6. Ne 58095
7. Risedronic Acid Monosodium Salt
8. Risedronate Monosodium
9. Ofg5exg60l
10. Risedronate Sodium Anhydrous
11. Ne-58095 Anhydrous
12. Sodium Trihydrogen (1-hydroxy-2-(3-pyridyl)ethylidene)diphosphonate
13. 115436-72-1 (sodium)
14. Risedronic Acid Sodium
15. Benet
16. Nsc-722598
17. Nsc-759280
18. Actonel (tn)
19. Phosphonic Acid, (1-hydroxy-2-(3-pyridinyl)ethylidene)bis-, Monosodium Salt
20. Sodium Hydrogen (1-hydroxy-1-phosphono-2-(pyridin-3-yl)ethyl)phosphonate
21. Risedronate Sodium [usan]
22. Risedronic Acid Sodium Salt
23. Mfcd00867080
24. 122458-82-6
25. Unii-ofg5exg60l
26. Optinate
27. Acrel
28. Risedronate Sodium [usan:usp]
29. Sodium Hydrogen [1-hydroxy-1-phosphono-2-(pyridin-3-yl)ethyl]phosphonate
30. Optinate Septimum
31. Monosodium Risedronate
32. Monosodium (1-hydroxy-2-(3-pyridinyl)ethylidene)bisphosphonate
33. Ne 58095 Sodium
34. Risedronate Sodium (usp)
35. Chembl1654
36. Schembl18377
37. Risedronate Sodium, Min 97%
38. Chebi:8868
39. Dtxsid20924178
40. Risedronate Sodium [vandf]
41. Risedronate Sodium [mart.]
42. Act03362
43. Bcp22742
44. Hy-b0119
45. Risedronate Sodium [usp-rs]
46. Risedronate Sodium [who-dd]
47. Ac-732
48. Mfcd01706268
49. S1428
50. Akos015833432
51. Ab09807
52. Ccg-213235
53. Cs-1883
54. Nsc 722598
55. Nsc 759280
56. Risedronate Sodium [orange Book]
57. Ncgc00346521-01
58. Ac-20120
59. As-13309
60. Risedronate Sodium [usp Monograph]
61. Ft-0631078
62. M2289
63. Risedronic Acid Monosodium Salt [mi]
64. D00942
65. H11438
66. Ab01274790-01
67. Q-201723
68. Q27285633
69. 2-(3-pyridinyl)-2-hydroxyethylidene-1,1-bisphosphonate
70. Monosodium 1-hydroxy-2-(3-pyridyl)ethylidene-1,1-diphosphonate
71. 1-hydroxy-2-(3-pyridinyl) Ethylidene Bis-phosphonic Acid Monosodium Salt
72. Hydrogen [1-hydroxy-1-phosphono-2-(pyridin-3-yl)ethyl]phosphonate (na+)
73. P,p'-[1-hydroxy-2-(3-pyridonyl)ethylidene]bis-phosphonic Acid Sodium Salt
74. Phosphonic Acid, (1-hydroxy-2-(3-pyridinyl)ethylidene)bis-, Disodium Salt
75. Phosphonic Acid, (2-hydroxy-2-(3-pyridinyl)ethylidene)bis-, Disodium Salt
76. Risedronate Sodium, United States Pharmacopeia (usp) Reference Standard
77. Risedronate Sodium;risedronic Acid Sodium;ne-58095; Ne 58095; Ne58095
78. Sodium Hydrogen-1-hydroxy-1-phosphono-2-(pyridin-3-yl)ethylphosphonate
79. Sodium;hydroxy-(1-hydroxy-1-phosphono-2-pyridin-3-ylethyl)phosphinate
80. (1-hydroxy-2-(pyridin-3-yl)ethane-1,1-diyl)bis(phosphonic Acid) Sodium Salt
81. Risedronate Sodium 2.5-hydrate, European Pharmacopoeia (ep) Reference Standard
82. Risedronate Sodium, Pharmaceutical Secondary Standard; Certified Reference Material
83. 934544-85-1
| Molecular Weight | 305.09 g/mol |
|---|---|
| Molecular Formula | C7H10NNaO7P2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 304.98301994 g/mol |
| Monoisotopic Mass | 304.98301994 g/mol |
| Topological Polar Surface Area | 151 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 375 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Actonel |
| PubMed Health | Antacid, Calcium Containing (By mouth) |
| Drug Classes | Antacid, Aluminum Containing, Antacid, Aluminum/Calcium/Magnesium Containing, Antacid, Calcium Containing, Antacid, Magnesium Containing, Antiflatulent, Calcium Supplement |
| Drug Label | ACTONEL (risedronate sodium) tablets is a pyridinyl bisphosphonate that inhibits osteoclast-mediated bone resorption and modulates bone metabolism. Each ACTONEL tablet for oral administration contains the equivalent of 5, 30, 35, 75, or 150 mg of anh... |
| Active Ingredient | Risedronate sodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 30mg; 150mg; 35mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 2 of 6 | |
|---|---|
| Drug Name | Atelvia |
| Drug Label | Atelvia (risedronate sodium) delayed-release tablets contain a pH-sensitive enteric coating and a chelating agent (EDTA). Risedronate is a pyridinyl bisphosphonate that inhibits osteoclast-mediated bone resorption and modulates bone metabolism. Each... |
| Active Ingredient | Risedronate sodium |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 35mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 3 of 6 | |
|---|---|
| Drug Name | Risedronate sodium |
| PubMed Health | Risedronate (By mouth) |
| Drug Classes | Calcium Regulator |
| Drug Label | ACTONEL (risedronate sodium) tablets is a pyridinyl bisphosphonate that inhibits osteoclast-mediated bone resorption and modulates bone metabolism. Each ACTONEL tablet for oral administration contains the equivalent of 5, 30, 35, 75, or 150 mg of anh... |
| Active Ingredient | Risedronate sodium |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 30mg; 150mg; 75mg; 5mg; 35mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Apotex; Aurobindo Pharma; Teva Pharms Usa; Sun Pharma Global; Teva Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Actonel |
| PubMed Health | Antacid, Calcium Containing (By mouth) |
| Drug Classes | Antacid, Aluminum Containing, Antacid, Aluminum/Calcium/Magnesium Containing, Antacid, Calcium Containing, Antacid, Magnesium Containing, Antiflatulent, Calcium Supplement |
| Drug Label | ACTONEL (risedronate sodium) tablets is a pyridinyl bisphosphonate that inhibits osteoclast-mediated bone resorption and modulates bone metabolism. Each ACTONEL tablet for oral administration contains the equivalent of 5, 30, 35, 75, or 150 mg of anh... |
| Active Ingredient | Risedronate sodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 30mg; 150mg; 35mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 5 of 6 | |
|---|---|
| Drug Name | Atelvia |
| Drug Label | Atelvia (risedronate sodium) delayed-release tablets contain a pH-sensitive enteric coating and a chelating agent (EDTA). Risedronate is a pyridinyl bisphosphonate that inhibits osteoclast-mediated bone resorption and modulates bone metabolism. Each... |
| Active Ingredient | Risedronate sodium |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 35mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 6 of 6 | |
|---|---|
| Drug Name | Risedronate sodium |
| PubMed Health | Risedronate (By mouth) |
| Drug Classes | Calcium Regulator |
| Drug Label | ACTONEL (risedronate sodium) tablets is a pyridinyl bisphosphonate that inhibits osteoclast-mediated bone resorption and modulates bone metabolism. Each ACTONEL tablet for oral administration contains the equivalent of 5, 30, 35, 75, or 150 mg of anh... |
| Active Ingredient | Risedronate sodium |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 30mg; 150mg; 75mg; 5mg; 35mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Apotex; Aurobindo Pharma; Teva Pharms Usa; Sun Pharma Global; Teva Pharms |
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?