
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
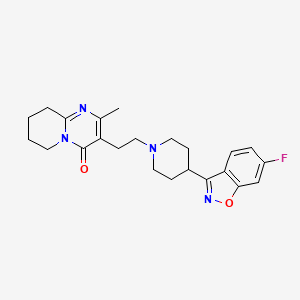

1. Consta, Risperdal
2. R 64,766
3. R 64766
4. R-64,766
5. R-64766
6. R64,766
7. R64766
8. Risperdal Consta
9. Risperidal
1. 106266-06-2
2. Risperdal
3. Risperidal
4. Rispolept
5. Risperdal Consta
6. Risperin
7. Rispolin
8. Sequinan
9. Apexidone
10. Risperidonum
11. Risperidona
12. Risperdal M-tab
13. R 64 766
14. Belivon
15. Psychodal
16. Spiron
17. Perseris
18. R 64766
19. R-64766
20. 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
21. Risperidone Impurity K
22. N05ax08
23. Chembl85
24. L6uh7zf8hc
25. 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
26. 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
27. Nsc-759895
28. R-64,766
29. R-64-766
30. Risperidal M-tab
31. Chebi:8871
32. Rcn3028
33. Risperidonum [latin]
34. Rcn-3028
35. Risperidona [spanish]
36. 106266-06-2 (free Base)
37. 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
38. Ncgc00015883-05
39. Ly-03004
40. Cas-106266-06-2
41. Dsstox_cid_25193
42. Dsstox_rid_80740
43. Dsstox_gsid_45193
44. 3-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one
45. 4h-pyrido(1,2-a)pyrimidin-4-one, 3-(2-(4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl)ethyl)-6,7,8,9-tetrahydro-2-methyl-
46. Zophrenal
47. R 64,766
48. Risperdal (tn)
49. 3-(2-(4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl)ethyl)-6,7,8,9-tetrahydro-2-methyl-4h-pyrido(1,2-a)pyrimidin-4-one
50. 3-[2-[4-(6-fluoranyl-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
51. 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
52. 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidino]ethyl}-6,7,8,9-tetrahydro-2-methylpyrido[1,2-a]pyrimidin-4-one
53. 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-4h,6h,7h,8h,9h-pyrido[1,2-a]pyrimidin-4-one
54. 4h-pyrido[1,2-a]pyrimidin-4-one, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-
55. Smr000466323
56. Sr-01000075399
57. Unii-l6uh7zf8hc
58. Mfcd00274576
59. Brn 4891881
60. Relday
61. Rispen
62. Okedi
63. Risperidone-ism
64. Hsdb 7580
65. Risperidone???
66. R64766
67. 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
68. 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl}-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
69. Risperidone [usan:usp:inn:ban]
70. Risperidone- Bio-x
71. 3-(2-(4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidino)ethyl)-6,7,8,9-tetrahydro-2-methyl-4h-pyrido(1,2-a)pyrimidin-4-one
72. Ks-1106
73. Risperidone (risperdal)
74. Lopac-r-118
75. Risperidone [mi]
76. Prestwick0_001029
77. Prestwick1_001029
78. Risperidone [inn]
79. Risperidone [jan]
80. R-118
81. Risperidone [hsdb]
82. Risperidone [usan]
83. Gtpl96
84. Risperidone [vandf]
85. Risperidone [mart.]
86. Risperidone(r 64 766)
87. Schembl27911
88. Risperidone [usp-rs]
89. Risperidone [who-dd]
90. Mls000759429
91. Mls001165758
92. Mls001424081
93. Us8802672, Risperidone
94. Bidd:gt0262
95. R 62 766
96. Spbio_003078
97. Risperidone (jp17/usp/inn)
98. Dtxsid8045193
99. Risperidone [ep Impurity]
100. Risperidone [orange Book]
101. Hms1571m19
102. Hms2051h07
103. Hms2089c22
104. Hms2098m19
105. Hms2233o11
106. Hms3373m18
107. Hms3393h07
108. Hms3657g13
109. Hms3715m19
110. Hms3887g15
111. Pharmakon1600-01506038
112. Risperidone [ep Monograph]
113. Risperidone [usp Impurity]
114. Zinc538312
115. Risperidone [usp Monograph]
116. Act04270
117. Bcp08161
118. Risperidone 1.0 Mg/ml In Methanol
119. Tox21_110253
120. Bdbm50001885
121. Cp-018
122. Nsc759895
123. Nsc786035
124. Nsc801188
125. S1615
126. Stk646402
127. Akos005577302
128. Tox21_110253_1
129. Ac-1306
130. Ccg-100930
131. Cs-1619
132. Db00734
133. Ly03004
134. Nc00180
135. Nsc 759895
136. Nsc-786035
137. Nsc-801188
138. Pb26023
139. Risperidone, >=98% (hplc), Powder
140. Ncgc00015883-01
141. Ncgc00015883-02
142. Ncgc00015883-03
143. Ncgc00015883-04
144. Ncgc00015883-06
145. Ncgc00015883-07
146. Ncgc00015883-08
147. Ncgc00015883-11
148. Ncgc00094352-01
149. Ncgc00094352-02
150. Ncgc00094352-03
151. Ncgc00179257-01
152. 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperi-dino]ethyl]-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]-pyrimidin-4-one
153. 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)-1-piperidyl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
154. 3-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
155. 4h-pyrido(1,2-a)pyrimidin-4-one, 6,7,8,9-tetrahydro-3-(2-(4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl)ethyl)-2-methyl-
156. Br164349
157. Hy-11018
158. Ab00514010
159. Eu-0101074
160. Ft-0631037
161. Ft-0674432
162. R0087
163. Sw197348-4
164. 66r062
165. D00426
166. Ab00514010-09
167. Ab00514010-11
168. Ab00514010-12
169. Ab00514010_13
170. Ab00514010_14
171. A801409
172. L000510
173. Q412443
174. J-001555
175. Sr-01000075399-2
176. Sr-01000075399-8
177. Brd-k53857191-001-04-5
178. Brd-k53857191-001-10-2
179. Z1522566617
180. Risperidone, British Pharmacopoeia (bp) Reference Standard
181. Risperidone, European Pharmacopoeia (ep) Reference Standard
182. Risperidone, United States Pharmacopeia (usp) Reference Standard
183. Risperidone, Pharmaceutical Secondary Standard; Certified Reference Material
184. Risperidone For System Suitability, European Pharmacopoeia (ep) Reference Standard
185. Risperidone Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
186. (risperidone)3-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one
187. 2-(2-(4-(benzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-7,8,9,9a-tetrahydro-1h-pyrido[1,2-a]pyrimidin-4(6h)-one
188. 2-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-6,7,8,8a-tetrahydro-5h-naphthalen-1-one
189. 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
190. 3-[2-[4(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
191. 3-[2-[4(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl4h-pyrido[1,2-a]pyrimidin-4-one
192. 3-[2-[4-(6 Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
193. 3-[2-[4-(6-fluoro- 1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl4h-pyrido[1,2-a]pyrimidin-4-one
194. 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidiny]ethyl]-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
195. 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-ethyl]-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
196. 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-me Thyl-4h-pyrido[1,2-a]pyrimidin-4-one
197. 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)-1-piperidyl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one;risperidone
198. 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[2,1-b]pyrimidin-4-one
199. 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-ethyl}- 6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
200. 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl}-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimid-in-4-one
201. 3-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one (resperidone)
202. 3-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one (risperidone)
203. 3-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one(risperidone)
204. 3-{2-[4-(6-fluorobenzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
205. 4h-pyrido[1,2-a]pyrimidin-4-one, 3-[2-[4-(6-fluoro-1,2-benz-isoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-
206. 5-[2-(4-benzo[d]isothiazol-3-yl-piperazin-1-yl)-ethyl]-6-methyl-1,3-dihydro-indol-2-one(norastemizole)
| Molecular Weight | 410.5 g/mol |
|---|---|
| Molecular Formula | C23H27FN4O2 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 410.21180428 g/mol |
| Monoisotopic Mass | 410.21180428 g/mol |
| Topological Polar Surface Area | 61.9 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 731 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Risperdal |
| PubMed Health | Risperidone (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | RISPERDAL contains risperidone, an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[... |
| Active Ingredient | Risperidone |
| Dosage Form | Tablet; Tablet, orally disintegrating; Solution |
| Route | Oral |
| Strength | 1mg/ml; 0.5mg; 1mg; 0.25mg; 4mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Risperdal consta |
| PubMed Health | Risperidone (Injection) |
| Drug Classes | Antipsychotic |
| Active Ingredient | Risperidone |
| Dosage Form | Injectable |
| Route | Intramuscular |
| Strength | 25mg/vial; 37.5mg/vial; 12.5mg/vial; 50mg/vial |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Risperidone |
| PubMed Health | Risperidone |
| Drug Classes | Antipsychotic |
| Drug Label | RISPERDAL contains risperidone, an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[... |
| Active Ingredient | Risperidone |
| Dosage Form | Tablet; Tablet, orally disintegrating; Solution |
| Route | oral; Oral |
| Strength | 1mg/ml; 0.5mg; 1mg; 0.25mg; 4mg; 2mg; 3mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Amneal Pharms; Wockhardt; Ranbaxy; Ani Pharms; West Ward Pharms; Actavis Labs Fl; Teva; Apotex; Aurobindo Pharma; Sun Pharm Inds; Taro; Torrent Pharms; Precision Dose; Sandoz; Prosam Labs; Prinston; Cipla; Par Pharm; Roxane; Watson Labs; Jub |
| 4 of 6 | |
|---|---|
| Drug Name | Risperdal |
| PubMed Health | Risperidone (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | RISPERDAL contains risperidone, an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[... |
| Active Ingredient | Risperidone |
| Dosage Form | Tablet; Tablet, orally disintegrating; Solution |
| Route | Oral |
| Strength | 1mg/ml; 0.5mg; 1mg; 0.25mg; 4mg; 2mg; 3mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Risperdal consta |
| PubMed Health | Risperidone (Injection) |
| Drug Classes | Antipsychotic |
| Active Ingredient | Risperidone |
| Dosage Form | Injectable |
| Route | Intramuscular |
| Strength | 25mg/vial; 37.5mg/vial; 12.5mg/vial; 50mg/vial |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Risperidone |
| PubMed Health | Risperidone |
| Drug Classes | Antipsychotic |
| Drug Label | RISPERDAL contains risperidone, an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[... |
| Active Ingredient | Risperidone |
| Dosage Form | Tablet; Tablet, orally disintegrating; Solution |
| Route | oral; Oral |
| Strength | 1mg/ml; 0.5mg; 1mg; 0.25mg; 4mg; 2mg; 3mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Amneal Pharms; Wockhardt; Ranbaxy; Ani Pharms; West Ward Pharms; Actavis Labs Fl; Teva; Apotex; Aurobindo Pharma; Sun Pharm Inds; Taro; Torrent Pharms; Precision Dose; Sandoz; Prosam Labs; Prinston; Cipla; Par Pharm; Roxane; Watson Labs; Jub |
Antipsychotic Agents; Dopamine Antagonists; Serotonin Antagonists
National Library of Medicine's Medical Subject Headings. Risperidone. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Risperdal (risperidone) is indicated for the treatment of schizophrenia. Efficacy was established in 4 short-term trials in adults, 2 short-term trials in adolescents (ages 13 to 17 years), and one long-term maintenance trial in adults /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Risperdal adjunctive therapy with lithium or valproate is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in one short-term trial in adults. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Risperdal is indicated for the treatment of irritability associated with autistic disorder, including symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods. Efficacy was established in 3 short-term trials in children and adolescents (ages 5 to 17 years). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Risperdal is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in 2 short-term trials in adults and one short-term trial in children and adolescents (ages 10 to 17 years). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
/BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Risperdal (risperidone) is not approved for the treatment of patients with dementia-related psychosis.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Like other antipsychotic agents (e.g., phenothiazines), risperidone has been associated with tardive dyskinesias. Although it has been suggested that atypical antipsychotics appear to have a lower risk of tardive dyskinesia, whether antipsychotic drugs differ in their potential to cause tardive dyskinesia is as yet unknown. In one open-label study, an annual incidence of tardive dyskinesia of 0.3% was reported in patients with schizophrenia who received approximately 8-9 mg of oral risperidone daily for at least 1 year. The prevalence of this syndrome appears to be highest among geriatric patients (particularly females). The risk of developing tardive dyskinesia and the likelihood that it will become irreversible also appear to increase with the duration of therapy and cumulative dose of antipsychotic agents administered; however, the syndrome may occur, although much less frequently, after relatively short periods of treatment with low dosages.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2514
Neuroleptic malignant syndrome (NMS), a potentially fatal symptom complex, has been reported in patients receiving antipsychotic agents. NMS requires immediate discontinuance of the drug and intensive symptomatic and supportive care.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2520
Dose-related somnolence was a commonly reported adverse effect associated with risperidone treatment. Approximately 8% of adult patients with schizophrenia receiving 16 mg of oral risperidone daily and 1% of patients receiving placebo reported somnolence in studies utilizing direct questioning or a checklist to detect adverse events, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2514
For more Drug Warnings (Complete) data for RISPERIDONE (41 total), please visit the HSDB record page.
Risperidone is indicated for the treatment of schizophrenia and irritability associated with autistic disorder. It is also indicated as monotherapy, or adjunctly with lithium or valproic acid, for the treatment of acute mania or mixed episodes associated with bipolar I disorder. Risperidone is additionally indicated in Canada for the short-term symptomatic management of aggression or psychotic symptoms in patients with severe dementia of the Alzheimer type unresponsive to nonpharmacological approaches. Risperidone is also used off-label for a number of conditions including as an adjunct to antidepressants in treatment-resistant depression.
FDA Label
Treatment of schizophrenia in adults for whom tolerability and effectiveness has been established with oral risperidone.
The primary action of risperidone is to decrease dopaminergic and serotonergic pathway activity in the brain, therefore decreasing symptoms of schizophrenia and mood disorders. Risperidone has a high binding affinity for serotonergic 5-HT2A receptors when compared to dopaminergic D2 receptors in the brain. Risperidone binds to D2 receptors with a lower affinity than first-generation antipsychotic drugs, which bind with very high affinity. A reduction in extrapyramidal symptoms with risperidone, when compared to its predecessors, is likely a result of its moderate affinity for dopaminergic D2 receptors.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
N05AX08
N05AX08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AX - Other antipsychotics
N05AX08 - Risperidone
Absorption
Well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution.
Route of Elimination
Risperidone is extensively metabolized in the liver. In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives are prolonged compared to young healthy subjects.
Volume of Distribution
The volume of distribution of risperidone is approximately 1 to 2 L/kg.
Clearance
Risperidone is cleared by the kidneys. Clearance is decreased in the elderly and those with a creatinine clearance (ClCr) between 15-59 mL/min, in whom clearance is decreased by approximately 60%.
Risperidone is well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Risperidone is rapidly distributed. The volume of distribution is 1-2 L/kg. In plasma, risperidone is bound to albumin and a1-acid glycoprotein. The plasma protein binding of risperidone is 90%, and that of its major metabolite, 9-hydroxyrisperidone, is 77%. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites. High therapeutic concentrations of sulfamethazine (100 ug/mL), warfarin (10 ug/mL), and carbamazepine (10 ug/mL) caused only a slight increase in the free fraction of risperidone at 10 ng/mL and 9-hydroxyrisperidone at 50 ng/mL, changes of unknown clinical significance.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Plasma concentrations of risperidone, its major metabolite, 9-hydroxyrisperidone, and risperidone plus 9-hydroxyrisperidone are dose proportional over the dosing range of 1 to 16 mg daily (0.5 to 8 mg twice daily). Following oral administration of solution or tablet, mean peak plasma concentrations of risperidone occurred at about 1 hour. Peak concentrations of 9-hydroxyrisperidone occurred at about 3 hours in extensive metabolizers, and 17 hours in poor metabolizers. Steady-state concentrations of risperidone are reached in 1 day in extensive metabolizers and would be expected to reach steady-state in about 5 days in poor metabolizers. Steady-state concentrations of 9-hydroxyrisperidone are reached in 5-6 days (measured in extensive metabolizers).
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Risperidone and 9-hydroxyrisperidone are present in human breast milk.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
For more Absorption, Distribution and Excretion (Complete) data for RISPERIDONE (6 total), please visit the HSDB record page.
Extensively metabolized by hepatic cytochrome P450 2D6 isozyme to 9-hydroxyrisperidone (i.e. [paliperidone]), which has approximately the same receptor binding affinity as risperidone. Hydroxylation is dependent on debrisoquine 4-hydroxylase and metabolism is sensitive to genetic polymorphisms in debrisoquine 4-hydroxylase. Risperidone also undergoes N-dealkylation to a lesser extent.
Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme, CYP 2D6. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone. CYP 2D6, also called debrisoquin hydroxylase, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP 2D6 is subject to genetic polymorphism (about 6%-8% of Caucasians, and a very low percentage of Asians, have little or no activity and are "poor metabolizers") and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP 2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP 2D6 metabolizers convert it much more slowly. Although extensive metabolizers have lower risperidone and higher 9-hydroxyrisperidone concentrations than poor metabolizers, the pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, are similar in extensive and poor metabolizers.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Risperidone has known human metabolites that include 3-[2-[4-(6-fluoro-2-hydroxy-1,2-benzoxazol-2-ium-3-yl)piperidin-1-yl]ethyl]-2,9-dimethyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one, 3-ethyl-2,9-dimethyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one, 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole, 9-Hydroxy-risperidone, and Paliperidone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
3 hours in extensive metabolizers Up to 20 hours in poor metabolizers
The apparent half-life of risperidone plus 9-hydroxyrisperidone following Risperdal Consta administration is 3 to 6 days, and is associated with a monoexponential decline in plasma concentrations. This half-life of 3-6 days is related to the erosion of the microspheres and subsequent absorption of risperidone.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL CONSTA (risperidone) kit (June 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bb34ee82-d2c2-43b8-ba21-2825c0954691
The apparent half-life of risperidone was 3 hours (CV=30%) in extensive metabolizers and 20 hours (CV=40%) in poor metabolizers. The apparent half-life of 9-hydroxyrisperidone was about 21 hours (CV=20%) in extensive metabolizers and 30 hours (CV=25%) in poor metabolizers. The pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, were similar in extensive and poor metabolizers, with an overall mean elimination half-life of about 20 hours.
US Natl Inst Health; DailyMed. Current Medication Information for RISPERDAL (risperidone) tablet RISPERDAL M-TAB (risperidone) tablet, orally disintegrating RISPERDAL (risperidone) solution (August 2012). Available from, as of November 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7e117c7e-02fc-4343-92a1-230061dfc5e0
Though its precise mechanism of action is not fully understood, current focus is on the ability of risperidone to inhibit the D2 dopaminergic receptors and 5-HT2A serotonergic receptors in the brain. Schizophrenia is thought to result from an excess of dopaminergic D2 and serotonergic 5-HT2A activity, resulting in overactivity of central mesolimbic pathways and mesocortical pathways, respectively. D2 dopaminergic receptors are transiently inhibited by risperidone, reducing dopaminergic neurotransmission, therefore decreasing positive symptoms of schizophrenia, such as delusions and hallucinations. Risperidone binds transiently and with loose affinity to the dopaminergic D2 receptor, with an ideal receptor occupancy of 60-70% for optimal effect. Rapid dissociation of risperidone from the D2 receptors contributes to decreased risk of extrapyramidal symptoms (EPS), which occur with permanent and high occupancy blockade of D2 dopaminergic receptors. Low-affinity binding and rapid dissociation from the D2 receptor distinguish risperidone from the traditional antipsychotic drugs. A higher occupancy rate of D2 receptors is said to increase the risk of extrapyramidal symptoms and is therefore to be avoided. Increased serotonergic mesocortical activity in schizophrenia results in negative symptoms, such as depression and decreased motivation. The high-affinity binding of risperidone to 5-HT2A receptors leads to a decrease in serotonergic activity. In addition, 5-HT2A receptor blockade results in decreased risk of extrapyramidal symptoms, likely by increasing dopamine release from the frontal cortex, and not the nigrostriatal tract. Dopamine level is therefore not completely inhibited. Through the above mechanisms, both serotonergic and D2 blockade by risperidone are thought to synergistically work to decrease the risk of extrapyramidal symptoms. Risperidone has also been said to be an antagonist of alpha-1 (1), alpha-2 (2), and histamine (H1) receptors. Blockade of these receptors is thought to improve symptoms of schizophrenia, however the exact mechanism of action on these receptors is not fully understood at this time.
Risperidone has high affinity for several receptors, including serotonin receptors (5-HT 2A/2C), D2 dopamine receptors and alpha1 and H1 receptors. It has no appreciable activity at M1 receptors. Its primary metabolite (9-hydroxyrisperidone) is nearly equipotent compared with the parent compound at D2 and 5-HT 2A receptors.
Goldfrank, L.R., Goldfrank's Toxicologic Emergencies 9th Ed. 2011., McGraw-Hill, New York, N.Y., p. 1006
The exact mechanism of antipsychotic action of risperidone has not been fully elucidated but, like that of clozapine, appears to be more complex than that of most other antipsychotic agents and may involve antagonism of central type 2 serotonergic (5-HT2) receptors and central dopamine D2 receptors.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2517
Risperidone is an atypical antipsychotic drug that is widely prescribed to young patients with different psychotic disorders. The long-term effects of this antipsychotic agent on neuronal receptors in developing brain remain unclear and require further investigation. In this study, we examined the effects of long-term treatment of risperidone on two serotonin receptor subtypes in brain regions of juvenile rat. Levels of 5-HT(1A) and 5-HT(2A) receptors in forebrain regions of juvenile rats were quantified after 3 weeks of treatment with three different doses of risperidone (0.3, 1.0 and 3.0mg/kg). Findings were compared to previously reported changes in 5-HT receptors after risperidone treatment (3.0mg/kg) in adult rat brain. The three doses of risperidone selectively and dose-dependently increased levels of 5-HT(1A) receptors in medial-prefrontal and dorsolateral-frontal cortices of juvenile animals. The higher doses (1.0 and 3.0mg/kg) of risperidone also increased 5-HT(1A) receptor binding in hippocampal CA(1) region of juvenile but not adult rats. In contrast, the three doses of risperidone significantly reduced 5-HT(2A) labeling in medial-prefrontal and dorsolateral-frontal cortices in juvenile as well as in adult animals in an equipotent fashion. 5-HT(1A) and 5-HT(2A) receptors in other forebrain regions were not altered by repeated risperidone treatment. These findings indicate that there are differential effects of risperidone on 5-HT(1A) and 5-HT(2A) receptors in juvenile animals, and that the 5-HT system in developing animals is more sensitive than adults to the long-term effects of risperidone.
PMID:19875272 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2819571 Choi YK et al; Eur Neuropsychopharmacol 20 (3): 187-94 (2010)
The main class of atypical antipsychotic drugs (APDs) in current use includes the protypical atypical APD, clozapine, as well as aripiprazole, asenapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone. At clinically effective doses, these agents produce extensive blockade of serotonin (5-HT)(2A) receptors, direct or indirect stimulation of 5-HT(1A) receptors, and to a lesser extent, reduction in dopamine (DA) D(2) receptor-mediated neurotransmission. This contrasts with typical APDs, for example haloperidol and perphenazine, which are mainly DA D(2/)D(3) receptor antagonists and have weaker, if any, potency as 5-HT(2A) receptor antagonists. Some, but not all, atypical APDs are also effective 5-HT(2C) receptor inverse agonists or neutral antagonists, 5-HT(6) or 5-HT(7) receptor antagonists. This diverse action on 5-HT receptors may contribute to significant differences in efficacy and tolerability among the atypical APDs. There is considerable preclinical and some clinical evidence that effects on 5-HT receptors contribute to the low risk of producing extrapyramidal side effects, which is the defining characteristic of an atypical APD, the lack of elevation in plasma prolactin levels (with risperidone and 9-hydroxyrisperidone being exceptions), antipsychotic action, and ability to improve some domains of cognition in patients with schizophrenia. The serotonergic actions of the atypical APDs, especially 5-HT(2A) receptor antagonism, are particularly important to the differential effects of typical and atypical APDs to overcome the effects of acute or subchronic administration of N-methyl-d-aspartate (NMDA) receptor antagonists, such as phencyclidine, ketamine, and dizocipline (MK-801). 5-HT(1A) receptor stimulation and 5-HT(6) and 5-HT(7) receptor antagonism may contribute to beneficial effects of these agents on cognition. In particular, 5-HT(7) receptor antagonism may be the basis for the pro-cognitive effects of the atypical APD, amisulpride, a D(2)/D(3) receptor antagonist, which has no effect on other 5-HT receptor. 5-HT(2C) receptor antagonism appears to contribute to the weight gain produced by some atypical APDs and may also affect cognition and psychosis via its influence on cortical and limbic dopaminergic activity.
PMID:21420906 Meltzer HY, Massey BW; Curr Opin Pharmacol 11 (1): 59-67 (2011)
Paliperidone is an active metabolite of the second-generation atypical antipsychotic, risperidone recently approved for the treatment of schizophrenia and schizoaffective disorder. Because paliperidone differs from risperidone by only a single hydroxyl group, questions have been raised as to whether there are significant differences in the effects elicited between these two drugs. /The researchers/ compared the relative efficacies of paliperidone versus risperidone to regulate several cellular signalling pathways coupled to four selected GPCR targets that are important for either therapeutic or adverse effects: human dopamine D2 , human serotonin 2A receptor subtype (5-HT2A ), human serotonin 2C receptor subtype and human histamine H1 receptors. Whereas the relative efficacies of paliperidone and risperidone were the same for some responses, significant differences were found for several receptor-signalling systems, with paliperidone having greater or less relative efficacy than risperidone depending upon the receptor-response pair. Interestingly, for 5-HT2A -mediated recruitment of beta-arrestin, 5-HT2A -mediated sensitization of ERK, and dopamine D2 -mediated sensitization of adenylyl cyclase signalling, both paliperidone and risperidone behaved as agonists. These results suggest that the single hydroxyl group of paliperidone promotes receptor conformations that can differ from those of risperidone leading to differences in the spectrum of regulation of cellular signal transduction cascades. Such differences in signalling at the cellular level could lead to differences between paliperidone and risperidone in therapeutic efficacy or in the generation of adverse effects.
PMID:23826915 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3791992 Clarke WP et al; Br J Pharmacol 170 (3): 532-45 (2013)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
25
PharmaCompass offers a list of Risperidone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Risperidone manufacturer or Risperidone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Risperidone manufacturer or Risperidone supplier.
PharmaCompass also assists you with knowing the Risperidone API Price utilized in the formulation of products. Risperidone API Price is not always fixed or binding as the Risperidone Price is obtained through a variety of data sources. The Risperidone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Risperidone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Risperidone, including repackagers and relabelers. The FDA regulates Risperidone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Risperidone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Risperidone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Risperidone supplier is an individual or a company that provides Risperidone active pharmaceutical ingredient (API) or Risperidone finished formulations upon request. The Risperidone suppliers may include Risperidone API manufacturers, exporters, distributors and traders.
click here to find a list of Risperidone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Risperidone DMF (Drug Master File) is a document detailing the whole manufacturing process of Risperidone active pharmaceutical ingredient (API) in detail. Different forms of Risperidone DMFs exist exist since differing nations have different regulations, such as Risperidone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Risperidone DMF submitted to regulatory agencies in the US is known as a USDMF. Risperidone USDMF includes data on Risperidone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Risperidone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Risperidone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Risperidone Drug Master File in Japan (Risperidone JDMF) empowers Risperidone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Risperidone JDMF during the approval evaluation for pharmaceutical products. At the time of Risperidone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Risperidone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Risperidone Drug Master File in Korea (Risperidone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Risperidone. The MFDS reviews the Risperidone KDMF as part of the drug registration process and uses the information provided in the Risperidone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Risperidone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Risperidone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Risperidone suppliers with KDMF on PharmaCompass.
A Risperidone CEP of the European Pharmacopoeia monograph is often referred to as a Risperidone Certificate of Suitability (COS). The purpose of a Risperidone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Risperidone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Risperidone to their clients by showing that a Risperidone CEP has been issued for it. The manufacturer submits a Risperidone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Risperidone CEP holder for the record. Additionally, the data presented in the Risperidone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Risperidone DMF.
A Risperidone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Risperidone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Risperidone suppliers with CEP (COS) on PharmaCompass.
A Risperidone written confirmation (Risperidone WC) is an official document issued by a regulatory agency to a Risperidone manufacturer, verifying that the manufacturing facility of a Risperidone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Risperidone APIs or Risperidone finished pharmaceutical products to another nation, regulatory agencies frequently require a Risperidone WC (written confirmation) as part of the regulatory process.
click here to find a list of Risperidone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Risperidone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Risperidone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Risperidone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Risperidone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Risperidone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Risperidone suppliers with NDC on PharmaCompass.
Risperidone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Risperidone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Risperidone GMP manufacturer or Risperidone GMP API supplier for your needs.
A Risperidone CoA (Certificate of Analysis) is a formal document that attests to Risperidone's compliance with Risperidone specifications and serves as a tool for batch-level quality control.
Risperidone CoA mostly includes findings from lab analyses of a specific batch. For each Risperidone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Risperidone may be tested according to a variety of international standards, such as European Pharmacopoeia (Risperidone EP), Risperidone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Risperidone USP).
