
Synopsis
Synopsis
0
VMF
0
Weekly News Recap #Phispers
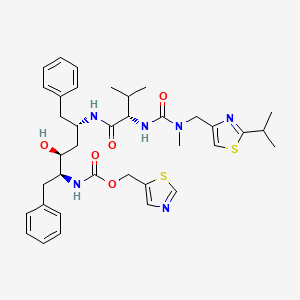

1. Abt 538
2. Abt-538
3. Abt538
4. Norvir
1. 155213-67-5
2. Norvir
3. Abt-538
4. A-84538
5. Abbott 84538
6. Abbott-84538
7. Empetus
8. Ritomune
9. Ritovir
10. Viekirax
11. Viriton
12. Rtv
13. Abt 538
14. Chebi:45409
15. 1,3-thiazol-5-ylmethyl N-[(2s,3s,5s)-3-hydroxy-5-[[(2s)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate
16. Nsc-693184
17. O3j8g9o825
18. Ritonavir Related Compounds Mixture
19. Nsc693184
20. 2,7,10,12-tetraazatridecanoic Acid, 4-hydroxy-12-methyl-9-(1-methylethyl)-13-[2-(1-methylethyl)-4-thiazolyl]-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl Ester, (3s,4s,6s,9s)-
21. Thiazol-5-ylmethyl ((2s,3s,5s)-3-hydroxy-5-((s)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanamido)-1,6-diphenylhexan-2-yl)carbamate
22. Ritonavir [usan]
23. Ncgc00159462-02
24. Ncgc00183130-01
25. Norvir Softgel
26. Dsstox_cid_28553
27. Dsstox_rid_82825
28. Dsstox_gsid_48627
29. N-[(2s,4s,5s)-4-hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl]-n~2~-(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)-l-valinamide
30. Rit
31. Abt538
32. Mfcd00927142
33. Drg-0244
34. 2,4,7,12-tetraazatridecan-13-oic Acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-(2-(1-methylethyl)-4-thiazolyl)-3,6-dioxo-8,11-bis(phenylmethyl)-, 5-thiazolylmethyl Ester, (5s-(5r*,8r*,10r*,11r*))-
35. Smr000466395
36. Thiazol-5-ylmethyl (2s,3s,5s)-3-hydroxy-5-((s)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanamido)-1,6-diphenylhexan-2-ylcarbamate
37. Thiazol-5-ylmethyl N-[(1s,2s,4s)-1-benzyl-2-hydroxy-4-[[(2s)-2-[[(2-isopropylthiazol-4-yl)methyl-methyl-carbamoyl]amino]-3-methyl-butanoyl]amino]-5-phenyl-pentyl]carbamate
38. Norvir (tm)
39. Norvir (tn)
40. Cas-155213-67-5
41. Hsdb 7160
42. 1,3-thiazol-5-ylmethyl N-[(2s,3s,5r)-3-hydroxy-5-[[(2s)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenyl-hexan-2-yl]carbamate
43. Ritonavirum
44. Tmc 114r
45. Unii-o3j8g9o825
46. 1hxw
47. 3prs
48. 3tne
49. 4eyr
50. Ritonavir [usan:usp:inn:ban]
51. Ritonavir- Bio-x
52. 2,4,7,12-tetraazatridecan-13-oic Acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-, 5-thiazolylmethyl Ester, [5s-(5r*,8r*,10r*,11r*)]-
53. 2,7,10,12-tetraazatridecanoic Acid, 4-hydroxy-12-methyl-9-(1-methylethyl)-13-(2-(1-methylethyl)-4-thiazolyl)-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl Ester, (3s,4s,6s,9s)-
54. Ritonavir & Plga
55. 5-thiazolylmethyl ((alphas)-alpha-((1s,3s-1-hydroxy-3-((2s)-2-(3-((2-isopropyl-4-thiazolyl)methyl)-3-methylureido)-3-methylbutyramido)-4-phenylbutyl)phenethyl)carbamate
56. Norvir, Norvir Softgel
57. 1sh9
58. Ritonavir [inn]
59. Ritonavir [jan]
60. Ritonavir [mi]
61. Ritonavir [hsdb]
62. Ritonavir [vandf]
63. Abbot 84538
64. Chembl163
65. Ritonavir [mart.]
66. Ritonavir [usp-rs]
67. Ritonavir [who-dd]
68. Ritonavir [who-ip]
69. Schembl6679
70. Ritonavir (jan/usp/inn)
71. Bidd:pxr0023
72. Ritonavir [ema Epar]
73. 5-thiazolylmethyl ((alphas)-alpha-((1s,3s)-1-hydroxy-3-((2s)-2-(3-((2-isopropyl-4-thiazolyl)methyl)-3-methylureido)-3-methylbutyramido)-4-phenylbutyl)phenethyl)carbamate
74. Mls000759541
75. Mls001424063
76. Mls006011764
77. Bidd:gt0387
78. Gtpl8804
79. Ritonavir [orange Book]
80. Dtxsid1048627
81. Ritonavir [usp Impurity]
82. Ritonavir, >=98% (hplc)
83. Ritonavir [usp Monograph]
84. Kaletra Component Ritonavir
85. Hms2051b08
86. Hms2235o10
87. Hms3715l22
88. Pharmakon1600-01502391
89. Paxlovid Component Ritonavir
90. Ritonavirum [who-ip Latin]
91. Viekirax Component Ritonavir
92. Zinc3944422
93. Tox21_112969
94. Tox21_113431
95. Ac-733
96. Bdbm50088504
97. Nsc760369
98. S1185
99. Stk634209
100. Ritonavir Component Of Kaletra
101. Akos000280930
102. Ritonavir & Poly-lactide-co-glycolide
103. Ritonavir Component Of Viekirax
104. Tox21_112969_1
105. Ccg-101007
106. Cs-0432
107. Db00503
108. Ks-5017
109. Nc00257
110. Nsc 693184
111. Nsc 760369
112. Nsc-760369
113. Mrf-0000287
114. Ncgc00159462-03
115. Ncgc00159462-04
116. Ncgc00159462-07
117. Ncgc00159462-20
118. Ritonavir 100 Microg/ml In Acetonitrile
119. Br164353
120. Hy-90001
121. Mls000759541-02
122. R0116
123. Sw197637-2
124. C07240
125. D00427
126. Ab00639991-06
127. Ab00639991-08
128. Ab00639991_09
129. Ab00639991_10
130. 213r675
131. A 84538
132. A904691
133. Q422618
134. J-009178
135. Ritonavir Related Compounds Mixture [usp-rs]
136. Brd-k51485625-001-07-6
137. Ritonavir Solution, 1.0 Mg/ml In Acetonitrile, Certified Reference Material
138. (2s, 3s, 5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinvl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
139. (2s,3s,5s )-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl) Methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl) Methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
140. (2s,3s,5s)-5-(n-(n((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
141. (2s,3s,5s)-5-(n-(n-((n-methyi-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1.6-diphenyl-3-hydroxyhexane
142. (2s,3s,5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl) Methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
143. (2s,3s,5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino )-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
144. (2s,3s,5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1 .6-diphenyl-3-hydroxyhexane
145. (2s,3s,5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
146. (2s,3s,5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1,6-diphenyl-3hydroxyhexane
147. (2s,3s,5s)-5-(n-(n-((n-methyl-n-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)valinyl)amino)-2-(n-((5-thiazolyl)methoxycarbonyl)amino)-1.6-diphenyl-3-hydroxyhexane
148. 1,3-thiazol-5-ylmethyl N-[(2s,3s,5s)-3-hydroxy-5-[(2s)-3-methyl-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}butanamido]-1,6-diphenylhexan-2-yl]carbamate
149. 1,3-thiazol-5-ylmethyl N-[(2s,3s,5s)-3-hydroxy-5-[[(2s)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-di(phenyl)hexan-2-yl]carbamate
150. 2,4,7,12-tetraazatridecan-13-oic Acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-(2-(1-methylethyl)-4-thiazolyl)-3,6-dioxo-8,11-bis(phenylmethyl)-5-thiazolylmethyl Ester (5s-(5r*,8r*,10r*,11r*))-
151. 5-thiazolylmethyl ((.alpha.s)-.alpha.-((1s,3s)-1-hydroxy-3-((2s)-2-(3-((2-isopropyl-4-thiazolyl)methyl)-3-methylureido)-3-methylbutyramido)-4-phenylbutyl)phenethyl)carbamate
152. 5-thiazolylmethyl (3s,4s,6s,9s)-4-hydroxy-12-methyl-9-(1-methylethyl)-13-[2-(1-methylethyl)-4-thiazolyl]-8,11-dioxo-3,6-bis(phenylmethyl)-2,7,10,12-tetraazatridecanoate
153. N-[(2s,4s,5s)-4-hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl]-n(2)-(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)-l-valinamide
154. N1-((1s,3s,4s)-1-benzyl-3-hydroxy-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl)-n2-{[[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino]carbonyl}-l-valinamide
155. Thiazol-5-ylmethyl((2s,3s,5s)-3-hydroxy-5-((s)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanamido)-1,6-diphenylhexan-2-yl)carbamate
| Molecular Weight | 720.9 g/mol |
|---|---|
| Molecular Formula | C37H48N6O5S2 |
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 18 |
| Exact Mass | 720.31276100 g/mol |
| Monoisotopic Mass | 720.31276100 g/mol |
| Topological Polar Surface Area | 202 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 1040 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Norvir |
| PubMed Health | Ritonavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | NORVIR (ritonavir) is an inhibitor of HIV-1 protease with activity against the Human Immunodeficiency Virus (HIV) type 1.Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis... |
| Active Ingredient | Ritonavir |
| Dosage Form | Tablet; Capsule; Solution |
| Route | Oral |
| Strength | 80mg/ml; 100mg |
| Market Status | Prescription |
| Company | Abbvie |
| 2 of 4 | |
|---|---|
| Drug Name | Ritonavir |
| PubMed Health | Ritonavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | NORVIR (ritonavir) is an inhibitor of HIV-1 protease with activity against the Human Immunodeficiency Virus (HIV) type 1.Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis... |
| Active Ingredient | Ritonavir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg |
| Market Status | Tentative Approval |
| Company | Roxane |
| 3 of 4 | |
|---|---|
| Drug Name | Norvir |
| PubMed Health | Ritonavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | NORVIR (ritonavir) is an inhibitor of HIV-1 protease with activity against the Human Immunodeficiency Virus (HIV) type 1.Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis... |
| Active Ingredient | Ritonavir |
| Dosage Form | Tablet; Capsule; Solution |
| Route | Oral |
| Strength | 80mg/ml; 100mg |
| Market Status | Prescription |
| Company | Abbvie |
| 4 of 4 | |
|---|---|
| Drug Name | Ritonavir |
| PubMed Health | Ritonavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | NORVIR (ritonavir) is an inhibitor of HIV-1 protease with activity against the Human Immunodeficiency Virus (HIV) type 1.Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis... |
| Active Ingredient | Ritonavir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg |
| Market Status | Tentative Approval |
| Company | Roxane |
Ritonavir is indicated in combination with nucleoside analogs or as monotherapy for the treatment of HIV infection or AIDS. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2401
Lopinavir/ritonavir has demonstrated antiviral activity in the HIV-infected adult. The objective of this study was to investigate a liquid coformulation of lopinavir/ritonavir, in combination with reverse transcriptase inhibitors, in HIV-infected children. One hundred antiretroviral (ARV)-naive and ARV-experienced, nonnucleoside reverse transcriptase inhibitor-naive children between 6 months and 12 years of age participated in this Phase I/II, open label, multicenter trial. Subjects initially received either 230/57.5 mg/sq m or 300/75 mg/sq m lopinavir/ritonavir twice daily; ARV-naive subjects also received stavudine and lamivudine, whereas ARV-experienced subjects also received nevirapine and one or two nucleoside reverse transcriptase inhibitors. Lopinavir/ritonavir pharmacokinetics, safety and efficacy were evaluated. All subjects were escalated to the 300/75 mg/sq m twice daily dose based on results from an interim pharmacokinetic and safety evaluation. The pharmacokinetics of lopinavir did not appear to be dependent on age when dosing was based on body surface area but were decreased on coadministration with nevirapine. Overall 79% of subjects had HIV RNA levels <400 copies/mL at Week 48 (intent-to-treat: missing = failure). Mean increases in absolute and relative (percent) CD4 counts from baseline to Week 48 were observed in both ARV-naive subjects (404 cells/cu mm; 10.3%) and ARV-experienced subjects (284 cells/cu mm; 5.9%). Only one subject prematurely discontinued the study because of a study drug-related adverse event. The liquid coformulation of lopinavir/ritonavir demonstrated durable antiviral activity and was safe and well-tolerated after 48 weeks of treatment in HIV-infected children.
PMID:12634581 Saez-Llorens X et al; Pediatr Infect Dis J 22 (3): 216-24 (2003)
The most frequent adverse effects associated with ritonavir therapy involve the GI tract. In one clinical study in HIV-infected patients, nausea occurred in 25.6%, vomiting in 13.7%, diarrhea in 15.4%, taste perversion in 11.1%, abdominal pain in 6%, local throat irritation in 1.7%, anorexia in 1.7%, and flatulence in 0.9% of patients who received ritonavir monotherapy. In clinical studies in patients with HIV infection who received ritonavir in conjunction with nucleoside antiretroviral therapy or ritonavir in conjunction with saquinavir, nausea occurred in 18.4-46.6%, vomiting in 7.1-23.3%, diarrhea in 22.7-25%, taste perversion in 5-17.2%, anorexia in 4.3-8.6%, abdominal pain in 2.1-8.3%, local throat irritation in 0.9-2.8%, and flatulence in 1.7-3.5% of patients. Constipation, dyspepsia, or fecal incontinence occurred in 0.2-3.4, 0.7-5.9, or 2.8%, respectively, of patients receiving ritonavir with other antiretroviral agents; these effects were not reported in patients receiving ritonavir monotherapy. Many adverse GI effects reported with ritonavir are transient; vomiting persists for an average of 1 week, nausea for 2-3 weeks, and diarrhea for 5 weeks.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 689
Adverse GI effects reported in less than 2% of patients receiving ritonavir alone or in conjunction with other antiretroviral agents include abnormal stools, bloody diarrhea, cheilitis, cholangitis, colitis, dry mouth, dysphagia, enlarged abdomen, eructation, esophageal ulcer, esophagitis, gastritis, gastroenteritis, GI disorder, GI hemorrhage, gingivitis,ileus, melena, mouth ulcer, pseudomembranous colitis, rectal disorder, rectal hemorrhage, sialadenitis, stomatitis, taste loss, tenesmus, thirst, tongue edema, and ulcerative colitis.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 689
Peripheral paresthesia occurred in 6% and paresthesia or circumoral paresthesia occurred in 2.6-3.4% of patients with HIV infection receiving ritonavir monotherapy in one clinical study (study 245). In clinical studies in patients receiving ritonavir in conjunction with nucleoside antiretroviral therapy (studies 245 and 247) or in conjunction with saquinavir (study 462), peripheral paresthesia was reported in 55.7%, paresthesia in 2.1-5.2%, and circumoral paresthesia in 5.2-6.7% of patients. Asthenia occurred in 10.3% of patients receiving ritonavir monotherapy and in 15.3-28.4% of patients receiving ritonavir with other antiretroviral agents. Many of these adverse effects are transient; peripheral paresthesia persists for an average of 34 weeks and circumoral paresthesia and asthenia persist for 35 weeks.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 689
Dizziness, insomnia, or somnolence have been reported in 2.6% of patients receiving ritonavir monotherapy and in 3.9-8.5, 2-3.4, or 2.4-2.6%, respectively, of patients receiving ritonavir with other antiretroviral agents. Headache, depression, or abnormal thinking were reported in 4.3-7.8, 1.7-7.1, or 0.7-2.6%, respectively, of patients receiving ritonavir in conjunction with other antiretroviral agents. Anxiety or confusion were reported in up to 2.1% of patients receiving ritonavir with other antiretroviral agents.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 690
For more Drug Warnings (Complete) data for RITONAVIR (34 total), please visit the HSDB record page.
Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. Ritonavir is also authorized by the FDA for emergency use in Paxlovid - a COVID-19 antiviral treatment which includes [nirmatrelvir] - for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.
FDA Label
Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV 1 infected patients (adults and children of 2 years of age and older).
Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1-infected patients (adults and children of two years of age and older).
Ritonavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Ritonavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs. Modern protease inhibitors require the use of low-dose ritonavir to boost pharmacokinetic exposure through inhibition of metabolism via the cytochrome P450 3A4 enzyme pathway.
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
J05AE03
J05AE03
J05AE03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AE - Protease inhibitors
J05AE03 - Ritonavir
Absorption
The absolute bioavailability of ritonavir has not been determined. Following oral administration, peak concentrations are reached after approximately 2 hours and 4 hours (Tmax) after dosing under fasting and non-fasting conditions, respectively. It should be noted that ritonavir capsules and tablets are not considered bioequivalent.
Route of Elimination
Ritonavir is primarily eliminated in the feces. Following oral administration of a single 600mg dose of radiolabeled ritonavir, approximately 11.3 2.8% of the dose was excreted into the urine, of which 3.5 1.8% was unchanged parent drug. The same study found that 86.4 2.9% of the dose was excreted in the feces, of which 33.8 10.8% was unchanged parent drug.
Volume of Distribution
The estimated volume of distribution of ritonavir is 0.41 0.25 L/kg.
Clearance
The apparent oral clearance at steady-state is 8.8 3.2 L/h. Renal clearance is minimal and estimated to be <0.1 L/h.
Ritonavir and its metabolites are eliminated from the body predominantly in the feces (86% of unchanged drug and metabolites), with minor urinary elimination (11%, mostly metabolites).
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1369
Absorption of ritonavir is only slightly affected by diet, and this is somewhat dependent on the formulation. The overall absorption of ritonavir from the capsule formulation may increase by 15% when taken with meals. ... There is greater than sixfold variability in drug trough concentrations among patients given 600 mg of ritonavir every 12 hours.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1369
The extent of oral absorption is high and is not affected by food. Within the clinical concentration range, ritonavir is approximately 98 to 99% bound to plasma proteins, including albumin and alpha 1-acid glycoprotein. Cerebrospinal fluid (CSF) drug concentrations are low in relation to total plasma concentration. However, parallel decreases in the viral burden have been observed in the plasma, CSF and other tissues. ... About 34% and 3.5% of a 600 mg dose is excreted as unchanged drug in the feces and urine, respectively. The clinically relevant t1/2 beta is about 3 to 5 hours. Because of autoinduction, plasma concentrations generally reach steady state 2 weeks after the start of administration. The pharmacokinetics of ritonavir are relatively linear after multiple doses, with apparent oral clearance averaging 7 to 9 L/hr.
PMID:9812178 Hsu A et al; Clin Pharmacokinet 35 (4): 275-91 (1998)
Ritonavir is excreted principally in the feces, both as unchanged drug and metabolites. Following oral administration of 600 mg of radiolabeled ritonavir as the oral solution, 86.4% of the dose is excreted in feces (33.8% as unchanged drug) and 11.3% of the dose is excreted in urine (3.5% as unchanged drug).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 697
For more Absorption, Distribution and Excretion (Complete) data for RITONAVIR (6 total), please visit the HSDB record page.
Ritonavir circulates in the plasma predominantly as unchanged drug. Five metabolites have been identified. The isopropylthiazole oxidation metabolite (M-2) is the major metabolite in low plasma concentrations and retains similar antiviral activity to unchanged ritonavir. The cytochrome P450 enzymes CYP3A and CYP2D6 are the enzymes primarily involved in the metabolism of ritonavir.
... Ritonavir is primarily metabolised by cytochrome P450 (CYP) 3A isozymes and, to a lesser extent, by CYP2D6. Four major oxidative metabolites have been identified in humans, but are unlikely to contribute to the antiviral effect. ...
PMID:9812178 Hsu A et al; Clin Pharmacokinet 35 (4): 275-91 (1998)
Five ritonavir metabolites have been identified in human urine and feces. The isopropylthiazole oxidation metabolite (M2) appears to be the major metabolite. M2 (but not other metabolites) has antiviral activity similar to that of ritonavir; however, only very low concentrations of this metabolite are present in plasma. Other metabolites identified in in vitro studies include a decarbamoylated metabolite (M1) and a product of N-dealkylation at the urea terminus (M11).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 697
The approximate half-life of ritonavir is 3-5 hours.
The clinically relevant t1/2 beta is about 3 to 5 hours.
PMID:9812178 Hsu A et al; Clin Pharmacokinet 35 (4): 275-91 (1998)
Ritonavic inhibits the HIV viral proteinase enzyme that normally cleaves the structural and replicative proteins that arise from major HIV genes, such as *gag* and *pol*. *Gag* encodes proteins involved in the core and the nucleocapsid, while *pol* encodes the the HIV reverse transcriptase, ribonuclease H, integrase, and protease. The *pol*-encoded proteins are initially translated in the form of a larger precursoe polypeptide, *gag-pol*, and needs to be cleaved by HIV protease to form other complement proteins. Ritonavir prevents the cleavage of the *gag-pol* polyprotein, which results in noninfectious, immature viral particles. Ritonavir is a potent inhibitor of cytochrome P450 CYP3A4 isoenzyme present both in the intestinal tract and liver. It is a type II ligand that perfectly fits into the CYP3A4 active site cavity and irreversibly binds to the heme iron via the thiazole nitrogen, which decreases the redox potential of the protein and precludes its reduction with the redox partner, cytochrome P450 reductase. Ritonavir may also play a role in limiting cellular transport and efflux of other protease inhibitors via the P-glycoprotein and MRP efflux channels.
Unlike nucleoside antiretroviral agents, the antiviral activity of ritonavir does not depend on intracellular conversion to an active metabolite. Ritonavir and other HIV protease inhibitors (e.g., amprenavir, indinavir, lopinavir, nelfinavir, saquinavir) act at a different stage of the HIV replication cycle than nucleoside and nonnucleoside reverse transcriptase inhibitors, and results of in vitro studies indicate that the antiretroviral effects of HIV protease inhibitors and some nucleoside or nonnucleoside antiretroviral agents may be additive or synergistic.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 696
Ritonavir is a selective, competitive, reversible inhibitor of HIV protease. HIV protease, an aspartic endopeptidase that functions as a homodimer, plays an essential role in the HIV replication cycle and the formation of infectious virus. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes (i.e., p55 and p160) to form structural proteins of the virion core (i.e., p17, p24, p9, and p7) and essential viral enzymes (i.e., reverse transcriptase, integrase, and protease). By interfering with the formation of these essential proteins and enzymes, ritonavir blocks maturation of the virus and causes formation of nonfunctional, immature, noninfectious virions. Ritonavir is active in both acutely and chronically infected cells since it targets the HIV replication cycle after translation and before assembly. Thus, the drug is active in chronically infected cells (e.g., monocytes and macrophages) that generally are not affected by nucleoside reverse transcriptase inhibitors (e.g., didanosine, lamivudine, stavudine, zalcitabine, zidovudine). Ritonavir does not affect early stages of the HIV replication cycle; however, the drug interferes with production of infectious HIV and limits further infectious spread of the virus.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 696
While the complete mechanisms of antiviral activity of ritonavir have not been fully elucidated, ritonavir apparently inhibits replication of retroviruses, including human immunodeficiency virus type 1 (HIV-1) and 2 (HIV-2), by interfering with HIV protease. The drug, therefore, exerts a virustatic effect against retroviruses by acting as an HIV protease inhibitor.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 696

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
53
PharmaCompass offers a list of Ritonavir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ritonavir manufacturer or Ritonavir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ritonavir manufacturer or Ritonavir supplier.
PharmaCompass also assists you with knowing the Ritonavir API Price utilized in the formulation of products. Ritonavir API Price is not always fixed or binding as the Ritonavir Price is obtained through a variety of data sources. The Ritonavir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ritonavir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ritonavir, including repackagers and relabelers. The FDA regulates Ritonavir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ritonavir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ritonavir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ritonavir supplier is an individual or a company that provides Ritonavir active pharmaceutical ingredient (API) or Ritonavir finished formulations upon request. The Ritonavir suppliers may include Ritonavir API manufacturers, exporters, distributors and traders.
click here to find a list of Ritonavir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ritonavir DMF (Drug Master File) is a document detailing the whole manufacturing process of Ritonavir active pharmaceutical ingredient (API) in detail. Different forms of Ritonavir DMFs exist exist since differing nations have different regulations, such as Ritonavir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ritonavir DMF submitted to regulatory agencies in the US is known as a USDMF. Ritonavir USDMF includes data on Ritonavir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ritonavir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ritonavir suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Ritonavir Drug Master File in Japan (Ritonavir JDMF) empowers Ritonavir API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Ritonavir JDMF during the approval evaluation for pharmaceutical products. At the time of Ritonavir JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Ritonavir suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Ritonavir Drug Master File in Korea (Ritonavir KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Ritonavir. The MFDS reviews the Ritonavir KDMF as part of the drug registration process and uses the information provided in the Ritonavir KDMF to evaluate the safety and efficacy of the drug.
After submitting a Ritonavir KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Ritonavir API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Ritonavir suppliers with KDMF on PharmaCompass.
A Ritonavir CEP of the European Pharmacopoeia monograph is often referred to as a Ritonavir Certificate of Suitability (COS). The purpose of a Ritonavir CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ritonavir EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ritonavir to their clients by showing that a Ritonavir CEP has been issued for it. The manufacturer submits a Ritonavir CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ritonavir CEP holder for the record. Additionally, the data presented in the Ritonavir CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ritonavir DMF.
A Ritonavir CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ritonavir CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ritonavir suppliers with CEP (COS) on PharmaCompass.
A Ritonavir written confirmation (Ritonavir WC) is an official document issued by a regulatory agency to a Ritonavir manufacturer, verifying that the manufacturing facility of a Ritonavir active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ritonavir APIs or Ritonavir finished pharmaceutical products to another nation, regulatory agencies frequently require a Ritonavir WC (written confirmation) as part of the regulatory process.
click here to find a list of Ritonavir suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ritonavir as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ritonavir API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ritonavir as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ritonavir and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ritonavir NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ritonavir suppliers with NDC on PharmaCompass.
Ritonavir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ritonavir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ritonavir GMP manufacturer or Ritonavir GMP API supplier for your needs.
A Ritonavir CoA (Certificate of Analysis) is a formal document that attests to Ritonavir's compliance with Ritonavir specifications and serves as a tool for batch-level quality control.
Ritonavir CoA mostly includes findings from lab analyses of a specific batch. For each Ritonavir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ritonavir may be tested according to a variety of international standards, such as European Pharmacopoeia (Ritonavir EP), Ritonavir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ritonavir USP).
