
Synopsis
Synopsis
0
KDMF
0
VMF
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
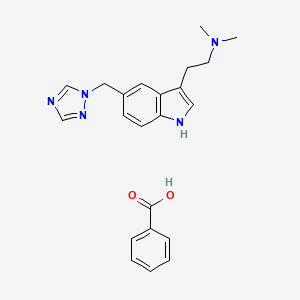

1. L 705,126
2. L 705126
3. L-705,126
4. L-705126
5. Maxalt
6. Mk 0462
7. Mk 462
8. Mk-0462
9. Mk-462
10. N,n-dimethyl-2-(5-(1,2,4-triazol-1-ylmethyl)-1h-indole-3-yl)ethylamine
11. Rizatriptan
1. 145202-66-0
2. Maxalt
3. Rizatriptan (benzoate)
4. Maxalt-mlt
5. Mk 462
6. Mk-462
7. Mk-0462
8. 2-(5-((1h-1,2,4-triazol-1-yl)methyl)-1h-indol-3-yl)-n,n-dimethylethanamine Benzoate
9. Nsc-758919
10. Wr978s7qhh
11. Rizatriptan Benzoate (maxalt)
12. N,n-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1h-indol-3-yl]ethylamine Benzoate
13. 3-(2-(dimethylamino)ethyl)-5-(1h-1,2,4-triazol-1-ylmethyl)indole Monobenzoate
14. 1h-indole-3-ethanamine, N,n-dimethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-, Monobenzoate
15. 2-(5-((1h-1,2,4-triazol-1-yl)methyl)-1h-indol-3-yl)-n,n-dimethylethan-1-amine Benzoate
16. Benzoic Acid;n,n-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1h-indol-3-yl]ethanamine
17. Rizatriptanbenzoate
18. N,n-dimethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-1h-indole-3-ethanamine Benzoate
19. Rizatriptan Benzoate [usan]
20. Mk 0462
21. Unii-wr978s7qhh
22. L 705126
23. L 705,126
24. L-705,126
25. Rizatriptan Benzoate [usan:usp]
26. Rizatrimptan Benzoate
27. Rizatriptane Benzoate
28. Rizatriptan, Benzoate
29. Mk-462 Benzoate
30. Schembl41226
31. Mls001424197
32. Chebi:8875
33. Rizatriptan Benzoate Salt
34. Chembl1201032
35. Rizatriptan Benzoate (jan/usp)
36. Amy8935
37. Dtxsid20162937
38. Rhb-103
39. Rizatriptan Benzoate [mi]
40. Hms2051b16
41. Hms2093k08
42. Hms2235m18
43. Hms3369i15
44. Hms3393b16
45. Hms3655k09
46. Hms3715d08
47. Hms3884k21
48. Pharmakon1600-01505189
49. Rizatriptan Benzoate [jan]
50. Act04340
51. Bcp02149
52. Hy-b0206
53. Int-0008
54. Rizatriptan Benzoate [vandf]
55. Ac-734
56. Mfcd00866224
57. Nsc758919
58. Rizatriptan Benzoate [mart.]
59. Rizatriptan Benzoate [usp-rs]
60. Rizatriptan Benzoate [who-dd]
61. Akos015855933
62. Ab07521
63. Ccg-101039
64. Ks-1189
65. Nc00289
66. Nsc 758919
67. Rizatriptan Benzoate [orange Book]
68. Smr000525252
69. Rizatriptan Benzoate [ep Monograph]
70. Db-015783
71. Rizatriptan Benzoate [usp Monograph]
72. Rizatriptan Benzoate Salt, >=98% (hplc)
73. Ft-0631171
74. R0107
75. R0181
76. S1607
77. Sw197669-2
78. D00675
79. 202r660
80. A808337
81. Sr-01000763588
82. J-008071
83. J-524222
84. Sr-01000763588-3
85. Q27292788
86. Rizatriptan Benzoate, European Pharmacopoeia (ep) Reference Standard
87. Rizatriptan Benzoate, United States Pharmacopeia (usp) Reference Standard
88. Dimethyl-[2-(5-[1,2,4]triazol-1-ylmethyl-1h-indol-3-yl)-ethyl]-amine Benzoate
89. N,n-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1h-indol-3-yl]ethanamine Benzoate
90. Rizatriptan For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 391.5 g/mol |
|---|---|
| Molecular Formula | C22H25N5O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 391.20082506 g/mol |
| Monoisotopic Mass | 391.20082506 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 412 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Maxalt |
| PubMed Health | Rizatriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | MAXALTCOPYRIGHT 1998, 2006 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved contains rizatriptan benzoate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist.Rizatriptan benzoate is described chemical |
| Active Ingredient | Rizatriptan benzoate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Merck |
| 2 of 4 | |
|---|---|
| Drug Name | Rizatriptan benzoate |
| Drug Label | MAXALTCOPYRIGHT 1998, 2006 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved contains rizatriptan benzoate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist.Rizatriptan benzoate is described chemical |
| Active Ingredient | Rizatriptan benzoate |
| Dosage Form | Tablet; Tablet, orally disintegrating |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Mylan Pharms; Apotex; Aurobindo Pharma; Natco Pharma; Sandoz; Invagen Pharms; Cipla; Sun Pharma Global; Glenmark Generics; Emcure Pharms; Teva Pharms; Macleods Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Maxalt |
| PubMed Health | Rizatriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | MAXALTCOPYRIGHT 1998, 2006 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved contains rizatriptan benzoate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist.Rizatriptan benzoate is described chemical |
| Active Ingredient | Rizatriptan benzoate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Merck |
| 4 of 4 | |
|---|---|
| Drug Name | Rizatriptan benzoate |
| Drug Label | MAXALTCOPYRIGHT 1998, 2006 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved contains rizatriptan benzoate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist.Rizatriptan benzoate is described chemical |
| Active Ingredient | Rizatriptan benzoate |
| Dosage Form | Tablet; Tablet, orally disintegrating |
| Route | Oral |
| Strength | eq 5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Mylan Pharms; Apotex; Aurobindo Pharma; Natco Pharma; Sandoz; Invagen Pharms; Cipla; Sun Pharma Global; Glenmark Generics; Emcure Pharms; Teva Pharms; Macleods Pharms |
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25623
Submission : 2011-12-22
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17633
Submission : 2004-08-30
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18021
Submission : 2005-01-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22676
Submission : 2009-03-31
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22763
Submission : 2009-05-08
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-06-02
Pay. Date : 2021-05-27
DMF Number : 20781
Submission : 2007-08-21
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-10-18
Pay. Date : 2013-05-24
DMF Number : 23066
Submission : 2009-08-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23581
Submission : 2010-05-28
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-09-11
Pay. Date : 2015-09-03
DMF Number : 19098
Submission : 2006-01-10
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23598
Submission : 2010-02-23
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Grade : Oral
Category : Co-Processed Excipients, Controlled & Modified Release, Direct Compression
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Controlled & Modified Release
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Film Forming Agent, Wet/Dry Granulation- Binder,Thickening & Suspension Agent, Non-Gelatin Capsule Manufacturing & Enteric Film Coating Systems
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Capsule, Orodispersible Tablet, Tablet
Grade : Not Available
Category : Controlled & Modified Release, Granulation, Taste Masking
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Sustained Release Tablet Matrix
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Application : Controlled & Modified Release
Excipient Details : These spheres can be used as an inert base for modified release formulations promoting consistency and uniformity of release profile thus ensuring a uniform therapeutic response.
Pharmacopoeia Ref : Particle Sizes - #20 – #30, ...
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Application : Controlled & Modified Release
Excipient Details : Instamodel Blend is used to provide Extended Release from the dosage form.
Dosage Form : Tablet
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Starch
Brand Name : HiCel MCC Spheres
Application : Controlled & Modified Release
Excipient Details : HiCel MCC Spheres are extremely versatile which is used for controlled release or sustained release formulations.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?