
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
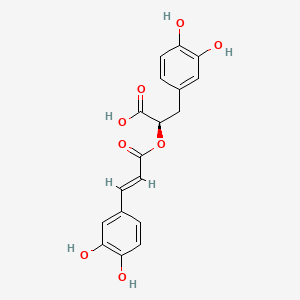

1. Rosmarinic Acid, (r-(e))-isomer
1. 20283-92-5
2. Rosemary Acid
3. (r)-rosmarinic Acid
4. Rosmarinicacid
5. Labiatic Acid
6. Labiatenic Acid
7. Trans-rosmarinic Acid
8. Rosmarinate
9. Rosmarinic Acid Racemate
10. Rosmarinic-acid
11. Meiji Red Perilla Polyphenol
12. 537-15-5
13. (2r)-3-(3,4-dihydroxyphenyl)-2-[(e)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxypropanoic Acid
14. (r,e)-3-(3,4-dihydroxyphenyl)-2-((3-(3,4-dihydroxyphenyl)acryloyl)oxy)propanoic Acid
15. Mqe6xg29yi
16. Chembl324842
17. Chebi:50371
18. (r)-o-(3,4-dihydroxycinnamoyl)-3-(3,4- Dihydroxyphenyl)lactic Acid
19. (2r)-3-(3,4-dihydroxyphenyl)-2-[(2e)-3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]propanoic Acid
20. (2r)-3-(3,4-dihydroxyphenyl)-2-{[(2e)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}propanoic Acid
21. Rosemaric Acid
22. (r)-o-(3,4-dihydroxycinnamoyl)-3-(3,4-dihydroxyphenyl)lactic Acid
23. (2r)-3-(3,4-dihydroxyphenyl)-2-[(e)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-propanoic Acid
24. Cinnamic Acid, 3,4-dihydroxy-, 2-ester With 3-(3,4-dihydroxyphenyl)lactic Acid
25. 3,4-dihydroxycinnamic Acid (r)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl Ester
26. Benzenepropanoic Acid, Alpha-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-
27. Unii-mqe6xg29yi
28. Rosmarimic Acid
29. Ccris 9361
30. Rm 21a
31. Hsdb 7688
32. Nplc 0542
33. R-(+)-2-(3,4-dihydroxycinnamoyloxy)-3-(3,4-dihydroxyphenyl)propionic Acid
34. Benzenepropanoic Acid, .alpha.-(((2e)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-, (.alpha.r)-
35. Benzenepropanoic Acid, .alpha.-[[(2e)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4-dihydroxy-, (.alpha.r)-
36. Mfcd00017740
37. Rosmarinic Acid, 2
38. Oristract Roa
39. Rosmarinic Acid, 96%
40. Bmse000648
41. Rosmarinic Acid [mi]
42. Mls000697677
43. Rosmarinic Acid [hsdb]
44. Rosmarinic Acid [inci]
45. Megxp0_000163
46. Rm-21a
47. Schembl1650675
48. Schembl2028694
49. Acon1_001068
50. Chebi:92370
51. Cid_5281792
52. Nplc-0542
53. Dtxsid20896987
54. Rosmarinic Acid [usp-rs]
55. Hms2227a13
56. Hms3266d13
57. Hms3411k16
58. Hms3649c22
59. Hms3675k16
60. Hms3885i15
61. Zinc899870
62. Hy-n0529
63. Bdbm50133496
64. S3612
65. Zb1872
66. 3,4-dihydroxycinnamic Acid 2-ester With 3-(3,4-dihydroxyphenyl)lactic Acid
67. Akos015892734
68. Ccg-207919
69. Ccg-208268
70. Ncgc00169708-01
71. Ac-33965
72. As-35341
73. Benzenepropanoic Acid,a-[[(2e)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4-dihydroxy-,(ar)-
74. Smr000445579
75. N1768
76. C01850
77. 225r532
78. A814378
79. Sr-01000946599
80. Q-100246
81. Sr-01000946599-1
82. (2r)-o-caffeoyl-3-(3,4-dihydroxyphenyl)lactic Acid
83. Q50380051
84. F0001-0715
85. Rosmarinic Acid (constituent Of Rosemary) [dsc]
86. 93b6a3bf-927d-4c59-8a49-29bdbc87c194
87. Rosmarinic Acid, Primary Pharmaceutical Reference Standard
88. Rosmarinic Acid (constituent Of Holy Basil Leaf) [dsc]
89. Rosmarinic Acid, European Pharmacopoeia (ep) Reference Standard
90. Rosmarinic Acid, >=98% (hplc), From Rosemarinus Officinalis L.
91. Rosmarinic Acid, United States Pharmacopeia (usp) Reference Standard
92. (r)-2-[3-(3,4-dihydroxyphenyl)acryloyloxy]-3-(3,4-dihydroxyphenyl)propionic Acid
93. (r,e)-3-(3,4-dihydroxyphenyl)-2-((3-(3,4-dihydroxyphenyl)acryloyl)oxy)propanoicacid
94. (r,e)-3-(3,4-dihydroxyphenyl)-2-(3-(3,4-dihydroxyphenyl)acryloyloxy)propanoic Acid
95. 3-(3,4-dihydroxyphenyl)acrylic Acid-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl Ester
96. Alpha-(((3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxybenzenepropanoic Acid
97. [r-(+)]-?-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4-dihydroxybenzenepropanoic Acid
98. Benzenepropanoic Acid, .alpha.-(((2e)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-3,4-dihydroxy-, (.alpha.r)-
99. Benzenepropanoic Acid, .alpha.-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-, (r-(e))-
100. Benzenepropanoic Acid, Alpha-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy-, (r-(e))-
| Molecular Weight | 360.3 g/mol |
|---|---|
| Molecular Formula | C18H16O8 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 360.08451746 g/mol |
| Monoisotopic Mass | 360.08451746 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Melissa officinalis L. (Lamiaceae) (lemon balm) is used in folk medicine for nervous complaints, lower abdominal disorders and, more recently, for treating Herpes simplex lesions. In this work the antiviral activity of a hydroalcoholic extract of lemon balm leaves against the Herpes simplex virus type 2 (HSV-2) was assessed by the cytopathic effect inhibition assay on Vero cells (ATCC CCL-81), in comparison with acyclovir. The cytotoxicity of the extract on Vero cells was previously tested by evaluating the cellular death and was confirmed by the Trypan blue test. Lemon balm showed to reduce the cytopathic effect of HSV-2 on Vero cells, in the range of non-toxic concentrations of 0.025-1 mg/mL (with reference to the starting crude herbal material). The maximum inhibiting effect (60%) was obtained with 0.5 mg/mL. The viral binding assay showed that the extract does not prevent the entry of HSV-2 in the cells, thus suggesting a mechanism of action subsequent to the penetration of the virus in the cell. The extract was also chemically characterized by NMR and HPLC analysis; it showed to contain cinnamic acid-like compounds, mainly rosmarinic acid (4.1% w/w). /The/ experiments support the use of lemon balm for treating Herpes simplex lesions and encourage clinical trials on this medicinal plant.
PMID:19023806 Mazzanti G et al; Nat Prod Res 22 (16): 1433-40 (2008)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Serine Proteinase Inhibitors
Exogenous or endogenous compounds which inhibit SERINE ENDOPEPTIDASES. (See all compounds classified as Serine Proteinase Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
The aim of this study in healthy humans was to determine the absorption, metabolism, and urinary excretion of rosmarinic acid (RA) after a single intake of perilla extract (PE). Six healthy men (mean age 37.2 +/- 6.2 y and mean body mass index 22.0 +/- 1.9 kg/sq m) were enrolled in the study that was a crossover design involving single intakes of PE containing 200 mg RA and placebo with a 10 day interval between treatments. Blood samples were collected before intake and at designated time intervals, while urine samples were collected over the periods 0-6 hr, 6-24 hr and 24-48 hr after intake. RA and its related metabolites in plasma and urine were measured by LC-MS. RA, methylated RA (methyl-RA), caffeic acid (CAA), ferulic acid (FA) and a trace of m-coumaric acid (COA) were detected in the urine after intake of PE. In plasma, RA, methyl-RA and FA were detected, with maximum levels obtained 0.5, 2 and 0.5 hr after intake of PE, respectively. The majority of these components in both plasma and urine were present as conjugated forms (glucuronide and/or sulfated). The proportion of RA and its related metabolites excreted in the urine was 6.3 +/- 2.2% of the total dose, with approximately 75% of these components being excreted within 6 hr after intake of PE.
PMID:15309457 Baba S et al; Eur J Nutr 44 (1): 1-9 (2005)
Rosmarinic acid is well absorbed from gastrointestinal tract and from the skin.
PMID:10641130 Al Sereiti et al; Indian J Exp Biol 37 (2): 124-30 (1999)
The purpose of the study was to investigate the transdermal absorption of rosmarinic acid (RA), its tissue distribution and absolute bioavailability. In ex vivo experiments, permeation of RA across excised rat skin was about 8 times higher from alcoholic solution than from water, indicating that ethanol may act as sorption promoter. The flux from water or alcoholic solution was 4.4 or 10 ug/sq cm/hr, and the tleg was 7.8 or 3.7 hr, respectively. After I.V. administration, RA is best described by a 2-compartment open model; t1/2 = 1.8 hr, t1/2 alpha = 0.07 hr, V tau = 2.3 L/kg, V beta = 15.3 L/kg. Upon topical administration of RA in form of a W/O ointment (25 mg/kg, 50 sq cm), the absolute bioavailability was 60%. 0.5 hours after iv administration, RA was detected and measured in brain, heart, liver, lung, muscle, spleen and bone tissue, showing the highest concentration in lung tissue (13 times the blood concentration), followed by spleen, heart and liver tissue. 4.5 hours (peak time) after topical administration of about 3 mg on the hind leg over 20 sq cm, RA was measured in blood, skin, muscle and bone tissue.
PMID:2755281 Ritschel WA et al; Methods Find Exp Clin Pharmacol 11 (5): 345-52 (1989)
The urine of rats administered rosmarinic acid (7) orally contained seven metabolites, which were identified as trans-caffeic acid 4-O-sulfate (1), trans-m-coumaric acid 3-O-sulfate (2), trans-ferulic acid 4-O-sulfate (3), trans-caffeic acid (4), m-hydroxyphenylpropionic acid (5), trans-m-coumaric acid (6), and unchanged rosmarinic acid (7) by spectroscopic and chemical data. The total cumulative amount of 1-7 excreted in the urine 48 h after the oral administration of rosmarinic acid was approximately 31.8% of the dose administered. On the other hand, the metabolites attributed to rosmarinic acid could not be found in the bile. Orally administered rosmarinic acid may thus be concluded to be excreted in the urine rather than in the bile, with cleavage of ester bonds, selective para-dehydroxylation, methylation, and sulfate-conjugation. Metabolites 2, 3, 5, and 6 were also detected in the plasma.
PMID:9722482 Nakazawa T, Ohsawa K; J Nat Prod 61 (8): 993-6 (1998)
Rosmarinic acid is the dominant hydroxycinnamic acid ester accumulated in Boraginaceae and Lamiaceae plants. A cytochrome P450 cDNA was isolated by differential display from cultured cells of Lithospermum erythrorhizon, and the gene product was designated CYP98A6 based on the deduced amino acid sequence. After expression in yeast, the P450 was shown to catalyze the 3-hydroxylation of 4-coumaroyl-4'-hydroxyphenyllactic acid, one of the final two steps leading to rosmarinic acid. The expression level of CYP98A6 is dramatically increased by addition of yeast extract or methyl jasmonate to L. erythrorhizon cells, and its expression pattern reflected the elicitor-induced change in rosmarinic acid production, indicating that CYP98A6 plays an important role in regulation of rosmarinic acid biosynthesis.
PMID:11943155 Matsuno M et al; FEBS Lett 514 (2-3): 219-24 (2002)
The aim of this study in healthy humans was to determine the absorption, metabolism, and urinary excretion of rosmarinic acid (RA) after a single intake of perilla extract (PE). Six healthy men (mean age 37.2 +/- 6.2 y and mean body mass index 22.0 +/- 1.9 kg/sq m) were enrolled in the study that was a crossover design involving single intakes of PE containing 200 mg RA and placebo with a 10 day interval between treatments. Blood samples were collected before intake and at designated time intervals, while urine samples were collected over the periods 0-6 hr, 6-24 hr and 24-48 hr after intake. RA and its related metabolites in plasma and urine were measured by LC-MS. RA, methylated RA (methyl-RA), caffeic acid (CAA), ferulic acid (FA) and a trace of m-coumaric acid (COA) were detected in the urine after intake of PE. In plasma, RA, methyl-RA and FA were detected, with maximum levels obtained 0.5, 2 and 0.5 hr after intake of PE, respectively. The majority of these components in both plasma and urine were present as conjugated forms (glucuronide and/or sulfated). The proportion of RA and its related metabolites excreted in the urine was 6.3 +/- 2.2% of the total dose, with approximately 75% of these components being excreted within 6 hr after intake of PE.
PMID:15309457 Baba S et al; Eur J Nutr 44 (1): 1-9 (2005)
... To determine the effects of rosmarinic acid on melanogenesis and elucidate the molecular events of melanogenesis induced by rosmarinic acid, several experiments were performed in B16 melanoma cells. In this study, ... the melanin content and tyrosinase expression were increased by rosmarinic acid in a concentration-dependent manner. In addition, after the melanin content was increased by rosmarinic acid, it was reduced by H-89 and KT 5720, protein kinase A (PKA) inhibitors, but not by SB203580, a p38mapk inhibitor, or Ro-32-0432, a PKC inhibitor, which suggests the involvement of PKA in rosmarinic acid-induced melanogenesis. Consistent with this, rosmarinic acid induced the phosphorylation of CRE-binding protein (CREB), but had no effect on the phosphorylation of p38mapk or the inhibition of Akt phosphorylation. Additionally, rosmarinic acid induced the activation of cAMP response element (CRE) without having any effect on cAMP production, which suggests that rosmarinic acid-induced melanogenesis is mediated by PKA, which occurs downstream of cAMP production. This result was further confirmed by the fact that rosmarinic acid-induced phosphorylation of CREB was inhibited by H-89, but not by PD98059, a MEK1 inhibitor, or by LY294002, a phosphatidylinositol-3-kinase (PI3K) inhibitor. Rosmarinic acid-induced expression of tyrosinase protein was attenuated by H-89. Based on these results, ... rosmarinic acid induces melanogenesis through PKA activation signaling.
PMID:17651699 Lee JS et al; Biochem Pharmacol 74 (7): 960-8 (2007)
Rosmarinic acid (RA) ... inhibits several complement-dependent inflammatory processes and may have potential as a therapeutic agent for the control of complement activation in disease. Rosmarinic acid has been reported to have effects on both the classical pathway C3-convertase and on the cobra venom factor-induced, alternative pathway convertase. In order to define the mechanism of inhibition, the effect of RA on classical and alternative pathway lysis, C1q binding, the classical pathway convertase, the alternative pathway convertase, membrane attack pathway lysis and the generation of fragments of C3 and C5 during activation, was tested in vitro. The results showed that RA inhibited lysis by the classical pathway more than by the alternative pathway. This effect was dose-dependent with maximum inhibition of classical pathway lysis observed at 2.6 mmoles of RA. There was little effect on C1q binding or on the classical and alternative pathway convertases. However, there was highly significant inhibition of lysis of pre-formed EA43b cells by dilutions of human or rabbit serum in the presence of RA (1 mM); this was accompanied by inhibition of C5a generation. /It was concluded/ that the inhibitory effect of RA involves the C5 convertase. Such inhibition could be advantageous to the host in disorders where the terminal attack sequence plays a role in pathogenesis.
PMID:1761351 Peake PW et al; Int J Immunopharmacol 13 (7): 853-7 (1991)
...Rosmarinic acid (RA), a naturally occurring polyphenol flavonoid, has been reported to inhibit TNF-alpha-induced NF-kappaB activation in human dermal fibroblasts. However, the precise mechanisms of RA have not been well elucidated in TNF-alpha-mediated anti-cancer therapy. In this study, /the authors/ found that RA treatment significantly sensitizes TNF-alpha-induced apoptosis in human leukemia U937 cells through the suppression of nuclear transcription factor-kappaB (NF-kappaB) and reactive oxygen species (ROS). Activation of caspases in response to TNF-alpha was markedly increased by RA treatment. However, pretreatment with the caspase-3 inhibitor, z-DEVD-fmk, was capable of significantly restoring cell viability in response to combined treatment. RA also suppressed NF-kappaB activation through inhibition of phosphorylation and degradation of IkappaBalpha, and nuclear translocation of p50 and p65. This inhibition was correlated with suppression of NF-kappaB-dependent anti-apoptotic proteins (IAP-1, IAP-2, and XIAP). RA treatment also normalized TNF-alpha-induced ROS generation. Additionally, ectopic Bcl-2 expressing U937 reversed combined treatment-induced cell death, cytochrome c release into cytosol, and collapse of mitochondrial potential. These results demonstrated that RA inhibits TNF-alpha-induced ROS generation and NF-kappaB activation, and enhances TNF-alpha-induced apoptosis.
PMID:19619938 Moon DO et al; Cancer Lett. 288(2):183-91 (2010)
Rosmarinic acid (RosA) is a hydroxylated compound frequently found in herbal plants and is mostly responsible for anti-inflammatory and antioxidative activity. Previously... RosA inhibited T-cell antigen receptor (TCR)- induced interleukin 2 (IL-2) expression and subsequent T-cell proliferation in vitro. /This study/ investigated /the/ inhibitory mechanism of RosA on TCR signaling, which ultimately activates IL-2 promoter by activating transcription factors, such as nuclear factor of activated T cells (NF-AT) and activating protein-1 (AP-1). Interestingly, RosA inhibited NF-AT activation but not AP-1, suggesting that RosA inhibits Ca+2-dependent signaling pathways only. Signaling events upstream of NF-AT activation, such as the generation of inositol 1,4,5-triphosphate and Ca+2 mobilization, and tyrosine phosphorylation of phospholipase C-gamma 1 (PLC-gamma 1) were strongly inhibited by RosA. Tyrosine phosphorylation of PLC-gamma 1 is largely dependent on 3 kinds of protein tyrosine kinases (PTKs), ie, Lck, ZAP-70, and Itk. /Investigators/ found that RosA efficiently inhibited TCR-induced tyrosine phosphorylation and subsequent activation of Itk but did not inhibit Lck or ZAP-70. ZAP-70-dependent signaling pathways such as the tyrosine phosphorylation of LAT and SLP-76 and serine/threonine phosphorylation of mitogen-activated protein kinases (MAPKs) were intact in the presence of RosA, confirming that RosA suppresses TCR signaling in a ZAP-70-independent manner. .../It is concluded/ that RosA inhibits TCR signaling leading to Ca+2 mobilization and NF-AT activation by blocking membrane-proximal events, specifically, the tyrosine phosphorylation of inducible T cells kinase (Itk) and PLC-gamma 1.
PMID:12511421 Kang MA et al; Blood 101(9):3534-42 (2003)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
19
PharmaCompass offers a list of Rosmarinic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rosmarinic Acid manufacturer or Rosmarinic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rosmarinic Acid manufacturer or Rosmarinic Acid supplier.
PharmaCompass also assists you with knowing the Rosmarinic Acid API Price utilized in the formulation of products. Rosmarinic Acid API Price is not always fixed or binding as the Rosmarinic Acid Price is obtained through a variety of data sources. The Rosmarinic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Rosmarinic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Rosmarinic Acid, including repackagers and relabelers. The FDA regulates Rosmarinic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Rosmarinic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Rosmarinic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Rosmarinic Acid supplier is an individual or a company that provides Rosmarinic Acid active pharmaceutical ingredient (API) or Rosmarinic Acid finished formulations upon request. The Rosmarinic Acid suppliers may include Rosmarinic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Rosmarinic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Rosmarinic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Rosmarinic Acid active pharmaceutical ingredient (API) in detail. Different forms of Rosmarinic Acid DMFs exist exist since differing nations have different regulations, such as Rosmarinic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Rosmarinic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Rosmarinic Acid USDMF includes data on Rosmarinic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Rosmarinic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Rosmarinic Acid suppliers with USDMF on PharmaCompass.
Rosmarinic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Rosmarinic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Rosmarinic Acid GMP manufacturer or Rosmarinic Acid GMP API supplier for your needs.
A Rosmarinic Acid CoA (Certificate of Analysis) is a formal document that attests to Rosmarinic Acid's compliance with Rosmarinic Acid specifications and serves as a tool for batch-level quality control.
Rosmarinic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Rosmarinic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Rosmarinic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Rosmarinic Acid EP), Rosmarinic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Rosmarinic Acid USP).

