
Synopsis
Synopsis
0
KDMF
0
VMF
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
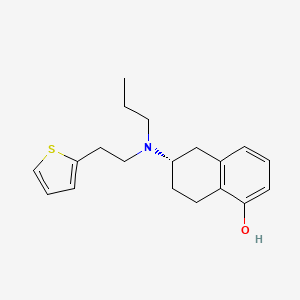

1. (+)-5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol
2. (+--)-5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol
3. 1-naphthalenol, 5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-
4. 2-(n-n-propyl-n-2-thienylethylamino)-5-hydroxytetralin
5. N 0437
6. N 0437, (+-)-isomer
7. N 0437, (-)-isomer
8. N 0437, (r)-isomer
9. N 0437, Hydrochloride, (r)-isomer
10. N 0437, Hydrochloride, (s)-isomer
11. N 0923
12. N 0924
13. N-0437
14. N-0923
15. N-0924
16. Neupro
17. Racemic N-0437
18. Rotigotine (+-)-form
19. Rotigotine Cds
20. Rotigotine, (+)-
21. Rotigotine, (+--)-
1. 99755-59-6
2. Neupro
3. Leganto
4. Spm 962
5. (s)-6-(propyl(2-(thiophen-2-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol
6. Spm-962
7. (6s)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol
8. N 0923
9. Chembl1303
10. (6s)-6-(propyl(2-(2-thienyl)ethyl)amino)-5,6,7,8-tetrahydro-1-naphthalenol
11. 87t4t8bo2e
12. 99755-59-6 (free Base)
13. N-0437
14. (-)-(s)-5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol
15. (6s)-5,6,7,8-tetrahydro-6-[propyl[2-(2-thienyl)ethyl]amino]-1-naphthalenol
16. Ncgc00168748-01
17. N-0923
18. Rotigotine Cds Patch
19. Dsstox_cid_26772
20. Dsstox_rid_81893
21. Dsstox_gsid_46772
22. (2s)-2-[propyl-[2-(2-thienyl)ethyl]amino]tetralin-5-ol
23. Rotigotine Cds
24. (s)-5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol
25. (s)-6-[propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-tetrahydro-naphthalen-1-ol
26. Rotigotine [inn]
27. Cas-99755-59-6
28. Neupro (tn)
29. Unii-87t4t8bo2e
30. Rotigotine [usan:inn:ban]
31. (s)-5,6,7,8-tetrahydro-6-[propyl[2-(2-thienyl)ethyl]amino]-1-naphthol
32. Spm-936
33. Spm-937
34. (6s)-rotigotine
35. Rotigotine [mi]
36. Rotigotine [jan]
37. Rotigotine [usan]
38. Rotigotine [vandf]
39. Rotigotine [mart.]
40. Rotigotine [usp-rs]
41. Rotigotine [who-dd]
42. Gtpl941
43. Rotigotine (jan/usan/inn)
44. Rotigotine [ema Epar]
45. (-)-n-0437
46. Schembl1088585
47. Zinc4028
48. Dtxsid5046772
49. Rotigotine [orange Book]
50. Hsdb 8254
51. Rotigotine [ep Monograph]
52. Chebi:135351
53. Hms3885d17
54. Rotigotine [usp Monograph]
55. Ex-a1164
56. Tox21_112627
57. Bdbm50123626
58. Mfcd00870193
59. S4274
60. Akos016340728
61. Tox21_112627_1
62. Ac-3547
63. Ccg-267650
64. Cs-0376
65. Db05271
66. Ss-4572
67. 1-naphthalenol, 5,6,7,8-tetrahydro-6-(propyl (2-(2-thienyl)ethyl)amino-(6s)-
68. Ncgc00168748-02
69. Hy-75502
70. R0220
71. Sw220014-1
72. D05768
73. F13103
74. 572r932
75. A846076
76. Q411985
77. J-502471
78. (6s)-6-(propyl-(2-thiophen-2-ylethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol
79. 1-naphthalenol, 5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino-(6s)-
| Molecular Weight | 315.5 g/mol |
|---|---|
| Molecular Formula | C19H25NOS |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 315.16568559 g/mol |
| Monoisotopic Mass | 315.16568559 g/mol |
| Topological Polar Surface Area | 51.7 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 337 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dopamine Agonists
National Library of Medicine's Medical Subject Headings. Rotigotine. Online file (MeSH, 2015). Available from, as of May 1, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Rotigotine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=Rotigotine
Neupro is indicated for the treatment of Parkinson's disease. /Included in US product label/
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
Neupro is indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome. /Included in US product label/
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
For more Therapeutic Uses (Complete) data for ROTIGOTINE (6 total), please visit the HSDB record page.
Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic behavior during Neupro treatment or after starting or increasing the dose of Neupro. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior may consist of one or more of the following: paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium. These various manifestations of psychotic behavior were also observed during the clinical development of Neupro for early- and advanced-stage Parkinson's disease and Restless Legs Syndrome.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
There was an increased risk for hallucinations in patients with advanced-stage Parkinson's disease treated with Neupro. In patients taking the maximum recommended Neupro dose, the incidence of hallucinations was 7% for Neupro and 3% for placebo, and this treatment difference increased with increasing dose. Hallucinations were of sufficient severity to cause discontinuation of treatment (mainly during the dose escalation/titration period) in 3% of advanced-stage Parkinson's disease patients treated with the maximum recommended dose of Neupro compared with 1% of placebo-treated patients. Hallucinations have also been reported in post-marketing reports.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
Patients with a major psychotic disorder should ordinarily not be treated with Neupro because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of Neupro.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
Patients may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including Neupro, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, or other urges while being treated with Neupro. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking Neupro.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
For more Drug Warnings (Complete) data for ROTIGOTINE (26 total), please visit the HSDB record page.
For use/treatment in neurologic disorders and parkinson's disease as well as moderate-to-severe primary Restless Legs Syndrome.
FDA Label
Leganto is indicated for the symptomatic treatment of moderate to severe idiopathic restless-legs syndrome in adults.
Leganto is indicated for the treatment of the signs and symptoms of early-stage idiopathic Parkinsons disease as monotherapy (i. e. without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end of dose or on-off fluctuations).
Parkinson's disease: Neupro is indicated for the treatment of the signs and symptoms of early-stage idiopathic Parkinson's disease as monotherapy (i. e. without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end of dose or 'on-off' fluctuations).
Restless-legs syndrome: Neupro is indicated for the symptomatic treatment of moderate to severe idiopathic restless-legs syndrome in adults.
Rotigotine is an agonist at all 5 dopamine receptor subtypes (D1-D5) but binds to the D3 receptor with the highest affinity. It is also an antagonist at -2-adrenergic receptors and an agonist at the 5HT1A receptors. Rotigotine also inhibits dopamine uptake and prolactin secretion. There is no indication of a QT/QTc prolonging effect of Neupro in doses up to 24 mg/24 hours. The effects of Neupro at doses up to 24 mg/24 hours (supratherapeutic doses) on the QT/QTc interval was evaluated in a double-blind, randomized, placebo- and positive-controlled (moxifloxacin 400 mg IV, single dose) parallel-group trial with an overall treatment period of 52 days in male and female patients with advanced-stage Parkinson's disease. Assay sensitivity was confirmed by significant QTc prolongation by moxifloxacin.
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
N04BC09
N04BC09
N - Nervous system
N04 - Anti-parkinson drugs
N04B - Dopaminergic agents
N04BC - Dopamine agonists
N04BC09 - Rotigotine
Absorption
Bioavailability varies depending on the application site. Differences in bioavailability were very small between the abdomen and hip (<1%). In contrast, the shoulder and thigh had a very large different in measured bioavailability (46%), with the shoulder showing the higher value. Tmax, 8 mg dose = 15 - 18 hours (it take approximately 3 hours until rotigotine reaches detectable levels in the plasma). The peak concentration cannot be observered. Steady state is reached in 2-3 days.
Route of Elimination
Urine (71%), Fecal (23%). Most of rotigotine that is excreted in the urine is in the form of inactive conjugates. Unchanged drug made up less <1%.
Volume of Distribution
The weight normalized apparent volume of distribution, (Vd/F), in humans is approximately 84 L/kg after repeated dose administration.
Results obtained with the patch administration in animals showed that the silicone based patch was superior to the acrylic based patch with respect to substance release. Following repeated dosing, 81 and 93 % substance was released from the silicone patch on the rat and monkey, respectively. The corresponding % release from the acrylic based patch was 28 and 22 %, respectively.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Neupro (Rotigotine), Scientific Discussion p.6 (2006). Available from, as of June 16, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000626/WC500026394.pdf
The weight normalized apparent volume of distribution (Vd/F) in humans is approximately 84 L/kg after repeated dose administration. The binding of rotigotine to human plasma proteins is approximately 92% in vitro and 89.5% in vivo.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
When single doses of 8 mg/24 hours are applied to the trunk, there is an average lag time of approximately 3 hours until drug is detected in plasma (range 1 to 8 hours). Tmax typically occurs between 15 to 18 hours post dose but can occur from 4 to 27 hours post dose. However, there is no characteristic peak concentration observed. Rotigotine displays dose-proportionality over a daily dose range of 1 mg/24 hours to 24 mg/24 hours. In the clinical studies of rotigotine effectiveness, the transdermal system application site was rotated from day to day (abdomen, thigh, hip, flank, shoulder, or upper arm) and the mean measured plasma concentrations of rotigotine were stable over the 6 months of maintenance treatment. Relative bioavailability for the different application sites at steady-state was evaluated in subjects with Parkinson's disease. In a single trial conducted in patients with early-stage Parkinson's disease, differences in bioavailability ranged from less than 1% (abdomen vs. hip) to 46% (shoulder vs. thigh) with shoulder application showing higher bioavailability.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
Rotigotine is primarily excreted in urine (approximately 71%) as inactive conjugates of the parent compound and N-desalkyl metabolites. A smaller proportion is excreted in feces (approximately 23%). The major metabolites found in urine were rotigotine sulfate (16% to 22% of the absorbed dose), rotigotine glucuronide (11% to 15%), and N-despropyl-rotigotine sulfate metabolite (14% to 20%) and N-desthienylethyl-rotigotine sulfate metabolite (10% to 21%). Approximately 11% is renally eliminated as other metabolites. A small amount of unconjugated rotigotine is renally eliminated (less than 1% of the absorbed dose).
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
For more Absorption, Distribution and Excretion (Complete) data for ROTIGOTINE (9 total), please visit the HSDB record page.
Hepatic (CYP-mediated). Rotigotine is extensively and rapidly metabolized by conjugation and N-dealkylation. After intravenous dosing the predominant metabolites in human plasma are sulfate conjugates of rotigotine, glucuronide conjugates of rotigotine, sulfate conjugates of the N-despropyl-rotigotine and conjugates of N-desthienylethyl-rotigotine. Multiple CYP isoenzymes, sulfotransferases and two UDP-glucuronosyltransferases catalyze the metabolism of rotigotine.
CYP2C19 was found to be the major CYP isoform involved in the phase 1 metabolism of rotigotine. However, multiple CYP-isoforms appear to be capable of catalyzing the metabolism. In vitro studies suggest a low risk for drug-drug interactions with co-administered drugs which are substrates of CYP isoforms in vivo. Also, no induction of human liver CYP isoforms has been found. No potential for displacement of rotigotine by warfarin and vice versa was detected with human serum albumin in vitro. Rotigotine was found not to be a substrate for P-glycoprotein and does not modulate digoxin transport in vitro.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Neupro (Rotigotine), Scientific Discussion p.7 (2006). Available from, as of June 16, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000626/WC500026394.pdf
Following absorption rotigotine was rapidly metabolised. Three phase 1 metabolites showed pharmacological activity. However, pharmacokinetics of these metabolites were not required as their presence in plasma was too low. The major metabolite observed in animal hepatocytes, the glucuronide conjugate of rotigotine, was in vivo excreted into bile and only reached the blood system at low levels. Conjugates of the Ndealkylated metabolites were found to be the major metabolites in plasma. Following subcutaneous administration, the sulfate and the glucuronide conjugates of the SPM 9206 metabolite and the sulfate conjugates of the SPM 9257 and the sulfate of the desthienylethyl despropyl metabolite were found to be the major metabolites in plasma. In human plasma, the sulphate conjugates of rotigotine, the SPM 9206 and the SPM 9257 metabolite were found to be the major metabolites. All the human major metabolites found in plasma were also found in the plasma of the main toxicological species.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Neupro (Rotigotine), Scientific Discussion p.7 (2006). Available from, as of June 16, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000626/WC500026394.pdf
Rotigotine is extensively metabolized by conjugation and N-dealkylation. After intravenous dosing the predominant metabolites in human plasma are sulfate conjugates of rotigotine, glucuronide conjugates of rotigotine, sulfate conjugates of the N-despropyl-rotigotine and conjugates of N-desthienylethyl-rotigotine. Multiple CYP isoenzymes, sulfotransferases and two UDP-glucuronosyltransferases catalyze the metabolism of rotigotine.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
Rotigotine is primarily excreted in urine (approximately 71%) as inactive conjugates of the parent compound and N-desalkyl metabolites. A smaller proportion is excreted in feces (approximately 23%). The major metabolites found in urine were rotigotine sulfate (16% to 22% of the absorbed dose), rotigotine glucuronide (11% to 15%), and N-despropyl-rotigotine sulfate metabolite (14% to 20%) and N-desthienylethyl-rotigotine sulfate metabolite (10% to 21%). Approximately 11% is renally eliminated as other metabolites. A small amount of unconjugated rotigotine is renally eliminated (less than 1% of the absorbed dose).
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
After removal of the patch, plasma levels decreased with a terminal half-life of 5 to 7 hours. The pharmacokinetic profile showed a biphasic elimination with an initial half-life of 3 hours.
After removal of the patch, plasma levels decreased with a terminal half-life of 5 to 7 hours. The pharmacokinetic profile showed a biphasic elimination with an initial half-life of 3 hours.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
... A single transdermal patch delivering 2 mg/24 hr rotigotine (patch content 4.5 mg) was applied to the ventral/lateral abdomen for 24 hr. ... The pharmacokinetic analysis included 48 subjects (24 Japanese, 24 Caucasian). ... The terminal half-life for unconjugated rotigotine was 5.3 hr in Japanese subjects and 5.7 hr in Caucasians; corresponding values for total rotigotine were 8.6 hr and 9.6 hr. ...
PMID:24178238 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3899448 Cawello W et al; Clin Drug Investig 34 (2): 95-105 (2014)
Rotigotine, a member of the dopamine agonist class of drugs, is delivered continuously through the skin (transdermal) using a silicone-based patch that is replaced every 24 hours. A dopamine agonist works by activating dopamine receptors in the body, mimicking the effect of the neurotransmitter dopamine. The precise mechanism of action of rotigotine as a treatment for Restless Legs Syndrome is unknown but is thought to be related to its ability to stimulate dopamine
This review discusses the relationship between therapeutic plasma concentrations of antiparkinson dopamine agonists (rotigotine, pergolide, cabergoline, apomorphine, bromocriptine, ropinirole, pramipexole, and talipexole) and their in vitro pharmacology at dopamine D1, D2 and D3 receptors. A significant correlation was found between therapeutic plasma concentrations of these dopamine agonists and their agonist potencies (EC50) at D2 receptors, although no such correlation existed at D1 or D3 receptors, suggesting that D2 receptors could be the primary and common target for the antiparkinson action of all dopamine agonists. However, D1 receptor stimulation is also important for maintaining swallowing reflex, bladder function and cognition. In particular, continuous D1 and D2 receptor stimulation may be reduced to low levels among Parkinson's disease patients. Our findings revealed therapeutic plasma concentrations of rotigotine were similar to its agonist potencies at both D1 and D2 receptors. Thus, rotigotine may be beneficial for the treatment of Parkinson's disease patients in that this dopamine agonist has the potential of continuous stimulation of both D1 and D2 receptors in the clinical setting.
PMID:25536763 Tadori Y, Kobayashi H; Nihon Shinkei Seishin Yakurigaku Zasshi 34 (5-6): 127-32 (2014)
Rotigotine is a non-ergoline dopamine agonist. The precise mechanism of action of rotigotine as a treatment for Parkinson's disease is unknown, although it is thought to be related to its ability to stimulate dopamine receptors within the caudate-putamen in the brain. The precise mechanism of action of rotigotine as a treatment for Restless Legs Syndrome is unknown but is thought to be related to its ability to stimulate dopamine receptors.
NIH; DailyMed. Current Medication Information for Neupro (Rotigotine) Patch, Extended Release; Neupro (Rotigotine) (Updated: May 2015). Available from, as of June 16, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=939e28c5-f3a9-42c0-9a2d-8d471d82a6e0
In Parkinson disease the degeneration of dopaminergic neurones is believed to lead to a disinhibition of the subthalamic nucleus thus increasing the firing rate of the glutamatergic excitatory projections to the substantia nigra. In consequence, excessive glutamatergic activity will cause excitotoxicity and oxidative stress. In the present study we investigated mechanisms of glutamate toxicity and the neuroprotective potential of the dopamine agonist rotigotine towards dopaminergic neurones in mouse mesencephalic primary culture. Glutamate toxicity was mediated by the N-methyl-d-aspartic acid (NMDA) receptor and accompanied by a strong calcium influx into dopaminergic neurones for which the L-type voltage-sensitive calcium channels play an important role. The rate of superoxide production in the culture was highly increased. Deleterious nitric oxide production did not participate in glutamate-mediated excitotoxicity. Pretreatment of cultures with rotigotine significantly increased the survival of dopaminergic neurones exposed to glutamate. Rotigotine exerted its protective effects via dopamine receptor stimulation (presumably via dopamine D3 receptor) and decreased significantly the production of superoxide radicals. When cultures were preincubated with Phosphoinositol 3-Kinase (PI3K) inhibitors the protective effect of rotigotine was abolished suggesting a decisive role of the PI3K/Akt pathway in rotigotine-mediated neuroprotection. Consistently, exposure to rotigotine induced the activation of Akt by phosphorylation followed by phosphorylation, and thus inactivation, of the pro-apoptotic factor glycogen synthase kinase-3-beta (GSK-3-beta). Taken together, our work contributed to elucidating the mechanisms of glutamate toxicity in mesencephalic culture and unravelled the signalling pathways associated with rotigotine-induced neuroprotection against glutamate toxicity in primary dopaminergic cultures.
PMID:24365490 Oster S et al; Eur J Pharmacol 724: 31-42 (2014)
Rotigotine (Neupro) is a non-ergoline dopamine agonist developed for the once daily treatment of Parkinson's disease (PD) using a transdermal delivery system (patch) which provides patients with the drug continuously over 24 h. To fully understand the pharmacological actions of rotigotine, the present study determined its extended receptor profile. In standard binding assays, rotigotine demonstrated the highest affinity for dopamine receptors, particularly the dopamine D3 receptor (Ki=0.71 nM) with its affinities to other dopamine receptors being (Ki in nM): D4.2 (3.9), D4.7 (5.9), D5 (5.4), D2 (13.5), D4.4 (15), and D1 (83). Significant affinities were also demonstrated at alpha-adrenergic (alpha2B, Ki=27 nM) and serotonin receptors (5-HT1A Ki=30 nM). In newly developed reporter-gene assays for determination of functional activity, rotigotine behaved as a full agonist at dopamine receptors (rank order: D3>D2L>D1=D5>D4.4) with potencies 2,600 and 53 times higher than dopamine at dopamine D3 and D2L receptors, respectively. At alpha-adrenergic sites, rotigotine acted as an antagonist on alpha2B receptors. At serotonergic sites, rotigotine had a weak but significant agonistic activity at 5-HT1A receptors and a minor or nonexistent activity at other serotonin receptors. Thus, in respect to PD, rotigotine can be characterized as a specific dopamine receptor agonist with a preference for the D3 receptor over D2 and D1 receptors. In addition, it exhibits interaction with D4 and D5 receptors, the role of which in relation to PD is not clear yet. Among non-dopaminergic sites, rotigotine shows relevant affinity to only 5-HT1A and alpha2B receptors. Further studies are necessary to investigate the contribution of the different receptor subtypes to the efficacy of rotigotine in Parkinson's disease and possible other indications such as restless legs syndrome.
PMID:18704368 Scheller D et al; Naunyn Schmiedebergs Arch Pharmacol 379 (1): 73-86 (2009)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
47
PharmaCompass offers a list of Rotigotine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rotigotine manufacturer or Rotigotine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rotigotine manufacturer or Rotigotine supplier.
PharmaCompass also assists you with knowing the Rotigotine API Price utilized in the formulation of products. Rotigotine API Price is not always fixed or binding as the Rotigotine Price is obtained through a variety of data sources. The Rotigotine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Rotigotine Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Rotigotine Hydrochloride, including repackagers and relabelers. The FDA regulates Rotigotine Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Rotigotine Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Rotigotine Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Rotigotine Hydrochloride supplier is an individual or a company that provides Rotigotine Hydrochloride active pharmaceutical ingredient (API) or Rotigotine Hydrochloride finished formulations upon request. The Rotigotine Hydrochloride suppliers may include Rotigotine Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Rotigotine Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Rotigotine Hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Rotigotine Hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of Rotigotine Hydrochloride DMFs exist exist since differing nations have different regulations, such as Rotigotine Hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Rotigotine Hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. Rotigotine Hydrochloride USDMF includes data on Rotigotine Hydrochloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Rotigotine Hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Rotigotine Hydrochloride suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Rotigotine Hydrochloride Drug Master File in Japan (Rotigotine Hydrochloride JDMF) empowers Rotigotine Hydrochloride API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Rotigotine Hydrochloride JDMF during the approval evaluation for pharmaceutical products. At the time of Rotigotine Hydrochloride JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Rotigotine Hydrochloride suppliers with JDMF on PharmaCompass.
A Rotigotine Hydrochloride CEP of the European Pharmacopoeia monograph is often referred to as a Rotigotine Hydrochloride Certificate of Suitability (COS). The purpose of a Rotigotine Hydrochloride CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Rotigotine Hydrochloride EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Rotigotine Hydrochloride to their clients by showing that a Rotigotine Hydrochloride CEP has been issued for it. The manufacturer submits a Rotigotine Hydrochloride CEP (COS) as part of the market authorization procedure, and it takes on the role of a Rotigotine Hydrochloride CEP holder for the record. Additionally, the data presented in the Rotigotine Hydrochloride CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Rotigotine Hydrochloride DMF.
A Rotigotine Hydrochloride CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Rotigotine Hydrochloride CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Rotigotine Hydrochloride suppliers with CEP (COS) on PharmaCompass.
A Rotigotine Hydrochloride written confirmation (Rotigotine Hydrochloride WC) is an official document issued by a regulatory agency to a Rotigotine Hydrochloride manufacturer, verifying that the manufacturing facility of a Rotigotine Hydrochloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Rotigotine Hydrochloride APIs or Rotigotine Hydrochloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Rotigotine Hydrochloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Rotigotine Hydrochloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Rotigotine Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Rotigotine Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Rotigotine Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Rotigotine Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Rotigotine Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Rotigotine Hydrochloride suppliers with NDC on PharmaCompass.
Rotigotine Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Rotigotine Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Rotigotine Hydrochloride GMP manufacturer or Rotigotine Hydrochloride GMP API supplier for your needs.
A Rotigotine Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Rotigotine Hydrochloride's compliance with Rotigotine Hydrochloride specifications and serves as a tool for batch-level quality control.
Rotigotine Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Rotigotine Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Rotigotine Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Rotigotine Hydrochloride EP), Rotigotine Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Rotigotine Hydrochloride USP).

