
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
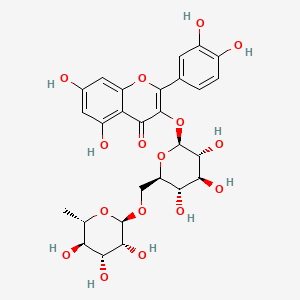

1. 3-rhamnosyl-glucosyl Quercetin
2. Quercetin 3 Rutinoside
3. Quercetin, 3-rhamnosyl-glucosyl
4. Quercetin-3-rutinoside
5. Rutoside
1. 153-18-4
2. Rutoside
3. Quercetin 3-rutinoside
4. Birutan
5. Phytomelin
6. Myrticolorin
7. Eldrin
8. Globulariacitrin
9. Rutin Trihydrate
10. Ilixanthin
11. Osyritrin
12. Paliuroside
13. Tanrutin
14. Sophorin
15. Bioflavonoid
16. Globularicitrin
17. Rutabion
18. Venoruton
19. 3-rutinosyl Quercetin
20. Violaquercitrin
21. Yunxianggan
22. 3-rhamnoglucosylquercetin
23. Rutine
24. Rutozyd
25. Melin
26. Quercetin 3-o-rutinoside
27. Rutinic Acid
28. Birutan Forte
29. Quercetin-3-rutinoside
30. Oxyritin
31. Rutinum
32. Rutosido
33. Rutosidum
34. Rutinion Acid
35. Vitamin P
36. Quercitin 3-rutinoside
37. Novarrutina
38. Violaquercetrin
39. Birutin
40. Quercetin Rhamnoglucosine
41. Quercetin-3-o-rutinoside
42. Quercetin-3beta-rutinoside
43. Quercetin 3-rhamnoglucoside
44. Quercetol 3-rhamnoglucoside
45. C.i. 75730
46. Rutosid
47. Rutoside [inn]
48. 3,3',4',5,7-pentahydroxyflavone-3-rutinoside
49. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-((((2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)methyl)tetrahydro-2h-pyran-2-yl)oxy)-4h-chromen-4-one
50. Quercetin 3-o-beta-d-rutinoside
51. 5g06tvy3r7
52. Chembl226335
53. Chebi:28527
54. Rutoside (inn)
55. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4h-chromen-3-yl 6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranoside
56. 3-[[6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4h-1-benzopyran-4-one
57. 3-rhamnoglucoside Of 3,3',4',5,7-pentahydroxyflavone
58. Ncgc00160628-01
59. Nsc-9220
60. Dsstox_cid_2326
61. Dsstox_rid_76549
62. Dsstox_gsid_22326
63. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[[(2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
64. 4h-1-benzopyran-4-one,3-[[6-o-(6-deoxy-a-l-mannopyranosyl)-b-d-glucopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-
65. Rut
66. Rutoside (rutin)
67. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[[(2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-chromen-4-one
68. 3-((6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranosyl)oxy)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4h-1-benzopyran-4-one
69. Cas-153-18-4
70. Smr000112560
71. Rutin [jan:nf]
72. Rutosidum [inn-latin]
73. Rutosido [inn-spanish]
74. Usaf Cf-5
75. Unii-5g06tvy3r7
76. Neoisorutin
77. Ccris 7564
78. Rutinoside, Quercetin-3, Beta-
79. Hydroxyethylrutoside
80. Rutin (rutoside)
81. Venoruton (tn)
82. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4h-chromen-3-yl 6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-mannopyranoside
83. Nsc 9220
84. Einecs 205-814-1
85. Mfcd00006830
86. Rutin,(s)
87. Brn 0075455
88. 3,3',4',5,7-pentahydroxyflavone 3-rutinoside
89. Ai3-19098
90. Rutin [vandf]
91. Rutin [inci]
92. 207671-50-9
93. Rutin [dsc]
94. Rutin [usp-rs]
95. Rutin [mi]
96. Rutoside [inn:jan:nf]
97. Rutoside [who-dd]
98. Beta-quercetin-3-rutinoside
99. Bidd:pxr0020
100. Schembl23243
101. 4h-1-benzopyran-4-one, 3-((6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranosyl)oxy)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-
102. 5-18-05-00519 (beilstein Handbook Reference)
103. Mls000759398
104. Mls001424098
105. Bidd:er0377
106. Divk1c_000644
107. Megxp0_000068
108. Rutin From Sophora Japonica L.
109. Dtxsid3022326
110. Acon1_000075
111. Cid_5280805
112. Hms502a06
113. Kbio1_000644
114. Rutin (95%)
115. Ninds_000644
116. 3,3',4',5,7-pentahydroxyflavone-3-rhamnoglucoside
117. Hms2051b06
118. 3'4'5,7-tetoh-flavone-3-rut
119. Flavone, 3,3',4',5,7-pentahydroxy-, 3-(o-rhamnosylglucoside)
120. Hy-n0148
121. Zinc4096846
122. Quercetin 3-o-beta-delta-rutinoside
123. Tox21_111945
124. Tox21_202602
125. Bdbm50217942
126. Glucopyranoside, Quercetin-3 6-o-alpha-l-rhamnopyranosyl-, Beta-d
127. Quercetin, 3-(6-o-alpha-l-rhamnopyranosyl-beta-d-glucopyranoside)
128. S2350
129. 3,3',4',5,5',7-hexahydroxyflavone (6-o-alpha-l-rhamnosyl-beta-d-glucoside)
130. Akos015895432
131. Glucopyranoside, Quercetin-3 6-o-(6-deoxy-alpha-l-mannopyranosyl)-, Beta-d-
132. Quercetin, 3-(6-0-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranoside)
133. Tox21_111945_1
134. Ccg-100999
135. Cs-5573
136. Db01698
137. Ds-9708
138. Nc00249
139. Idi1_000644
140. Rutinoside, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4h-1-benzopyran-3-yl
141. Ncgc00160628-02
142. Ncgc00160628-03
143. Ncgc00260150-01
144. 4h-1-benzopyran-4-one, 3-[[6-o-(6-deoxy-.alpha.-l-mannopyranosyl)-.beta.-d-glucopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-
145. Methyltetrahydro-2h-pyran-2-yloxy)methyl)
146. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3
147. R0035
148. C05625
149. D08499
150. Ab00374708-09
151. Tetrahydro-2h-pyran-2-yloxy)-4h-chromen-4-one
152. A809400
153. Q407857
154. Sr-01000759399
155. Q-201691
156. Sr-01000759399-5
157. A8241da0-6ec6-48ba-bd10-3f7bd61d42ce
158. Brd-k20482099-001-01-1
159. Brd-k20482099-001-11-0
160. 3,3,4,5,7-pentahydroxyflavone-3-rhamnoglucoside
161. 3-rhamnoglucoside Of 5,7,3',4'-tetrahydroxyflavonol
162. Quercetin 3-o-[alpha-l-rhamnosyl-(1->6)-beta-d-glucoside]
163. Quercetin 3-o-.alpha.-l-rhamnopyranosyl-.beta.-d-glucopyranoside
164. Rutin (quercetin-3-o-rutinoside) (constituent Of Ginkgo) [dsc]
165. Rutin (quercetin-3-o-rutinoside) (constituent Of Hawthorn Leaf With Flower) [dsc]
166. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-((((2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)methyl)tetrahydro-2h-pyran-2-yl)oxy)-4h-chromen-4-one;rutin
167. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-((2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(((2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyltetrahydro-2h-pyran-2-yloxy)methyl)tetrahydro-2h-pyran-2-yloxy)-4h-chromen-4-one
168. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-({[(2r,3r,4r,5r,6s)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-4h-chromen-4-one
169. 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4h-chromen-4-one-3-yl 6-o-.alpha.-l-rhamnopyranosyl-.beta.-d-glucoside
170. 4h-1-benzopyran-4-one, 3-[[6-o-(6-deoxy-.alpha.-l-mannopyranosyl)-.beta.-d-glucopyranosyl]-oxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-
| Molecular Weight | 610.5 g/mol |
|---|---|
| Molecular Formula | C27H30O16 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 6 |
| Exact Mass | 610.15338487 g/mol |
| Monoisotopic Mass | 610.15338487 g/mol |
| Topological Polar Surface Area | 266 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1020 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
C05CA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C05 - Vasoprotectives
C05C - Capillary stabilizing agents
C05CA - Bioflavonoids
C05CA01 - Rutoside

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26727
Submission : 2012-11-05
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32
Submission : 1946-12-12
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26509
Submission : 2012-09-28
Status : Inactive
Type : II
Certificate Number : R0-CEP 2005-241 - Rev 00
Issue Date : 2008-09-29
Type : Chemical
Substance Number : 1795
Status : Withdrawn by Holder

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26287
Submission : 2012-06-28
Status : Inactive
Type : II


Certificate Number : CEP 2006-129 - Rev 03
Issue Date : 2024-08-21
Type : Chemical
Substance Number : 1795
Status : Valid


Certificate Number : CEP 2017-267 - Rev 02
Issue Date : 2024-11-15
Type : Chemical
Substance Number : 1795
Status : Valid


Certificate Number : R1-CEP 2004-155 - Rev 01
Issue Date : 2010-11-08
Type : Chemical
Substance Number : 1795
Status : Valid


Certificate Number : CEP 2011-383 - Rev 01
Issue Date : 2024-05-23
Type : Chemical
Substance Number : 1795
Status : Valid

Certificate Number : R0-CEP 2013-253 - Rev 00
Issue Date : 2014-10-15
Type : Chemical
Substance Number : 1795
Status : Expired

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Novos vital is a groundbreaking supplement chew scientifically formulated with an ingredient blend shown to support and optimize essential organ functions that decline with age.
Lead Product(s): Trehalose,Nattokinase,Rutin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Novos Vital
Study Phase: Approved FDFProduct Type: Carbohydrate
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Trehalose,Nattokinase,Rutin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
NOVOS Launches Daily Chew to Optimize and Maintain Essential Organ Function
Details : Novos vital is a groundbreaking supplement chew scientifically formulated with an ingredient blend shown to support and optimize essential organ functions that decline with age.
Product Name : Novos Vital
Product Type : Carbohydrate
Upfront Cash : Inapplicable
February 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Sun Pharma strengthen its anti-inflammatory portfolio by adding Disperzyme (bromelain), Disperzyme-CD and Phlogam from Aksigen Hospital Care.
Lead Product(s): Bromelain,Rutin,Trypsin
Therapeutic Area: Neurology Brand Name: Disperzyme
Study Phase: Approved FDFProduct Type: Enzyme
Sponsor: Sun Pharmaceutical Industries Limited
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition January 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bromelain,Rutin,Trypsin
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Sun Pharmaceutical Industries Limited
Deal Size : Undisclosed
Deal Type : Acquisition
Sun Pharma Acquires Disperzyme® and Phlogam® Brands to Strengthen Its Anti-Inflammatory Portfoli...
Details : Through the acquisition, Sun Pharma strengthen its anti-inflammatory portfolio by adding Disperzyme (bromelain), Disperzyme-CD and Phlogam from Aksigen Hospital Care.
Product Name : Disperzyme
Product Type : Enzyme
Upfront Cash : Undisclosed
January 30, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
69
PharmaCompass offers a list of Rutin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rutin manufacturer or Rutin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rutin manufacturer or Rutin supplier.
PharmaCompass also assists you with knowing the Rutin API Price utilized in the formulation of products. Rutin API Price is not always fixed or binding as the Rutin Price is obtained through a variety of data sources. The Rutin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Rutin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Rutin, including repackagers and relabelers. The FDA regulates Rutin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Rutin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Rutin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Rutin supplier is an individual or a company that provides Rutin active pharmaceutical ingredient (API) or Rutin finished formulations upon request. The Rutin suppliers may include Rutin API manufacturers, exporters, distributors and traders.
click here to find a list of Rutin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Rutin DMF (Drug Master File) is a document detailing the whole manufacturing process of Rutin active pharmaceutical ingredient (API) in detail. Different forms of Rutin DMFs exist exist since differing nations have different regulations, such as Rutin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Rutin DMF submitted to regulatory agencies in the US is known as a USDMF. Rutin USDMF includes data on Rutin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Rutin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Rutin suppliers with USDMF on PharmaCompass.
A Rutin CEP of the European Pharmacopoeia monograph is often referred to as a Rutin Certificate of Suitability (COS). The purpose of a Rutin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Rutin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Rutin to their clients by showing that a Rutin CEP has been issued for it. The manufacturer submits a Rutin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Rutin CEP holder for the record. Additionally, the data presented in the Rutin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Rutin DMF.
A Rutin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Rutin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Rutin suppliers with CEP (COS) on PharmaCompass.
Rutin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Rutin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Rutin GMP manufacturer or Rutin GMP API supplier for your needs.
A Rutin CoA (Certificate of Analysis) is a formal document that attests to Rutin's compliance with Rutin specifications and serves as a tool for batch-level quality control.
Rutin CoA mostly includes findings from lab analyses of a specific batch. For each Rutin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Rutin may be tested according to a variety of international standards, such as European Pharmacopoeia (Rutin EP), Rutin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Rutin USP).
