
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Canada

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
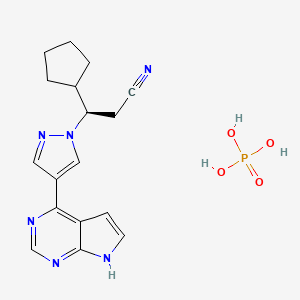

1. 3r)-3-cyclopentyl-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)pyrazol-1-yl)propanenitrile
2. Inc-424
3. Inc424
4. Inca24
5. Incb-018424
6. Incb-018424 Phosphate
7. Incb-018424 Salt
8. Incb-18424
9. Incb-18424 Phosphate
10. Incb018424
11. Incb018424 Phosphate
12. Jakafi
13. Jakavi
14. Opzelura
15. Ruxolitinib
16. Ruxolitinib (as Phosphate)
17. Ruxolitinib Monophosphate
1. 1092939-17-7
2. Jakafi
3. (r)-3-(4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl)-3-cyclopentylpropanenitrile Phosphate
4. Jakavi
5. Ruxolitinib (phosphate)
6. Ruxolitinib Monophosphate
7. Incb018424 Phosphate
8. Ruxolitinib (as Phosphate)
9. Incb-018424 Phosphate
10. Incb-018424 Salt
11. Ruxolitinib Phosphate [usan]
12. Incb-18424 Phosphate
13. Incb018424 Salt
14. Chebi:66917
15. 436lru32h5
16. 1092939-17-7 (phosphate)
17. (betar)-beta-cyclopentyl-4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazole-1-propanenitrile Phosphate
18. Incb-18424
19. Ruxolitinib Phosphate Salt
20. (3r)-3-cyclopentyl-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]propanenitrile Phosphate
21. (3r)-3-cyclopentyl-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)propanenitrile Phosphate (1:1)
22. Phosphenoperoxoic Acid Compound With (r)-3-(4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)- 1h-pyrazol-1-yl)-3-cyclopentylpropanenitrile And Dihydrogen (1:1:1)
23. Opzelura
24. Unii-436lru32h5
25. (3r)-3-cyclopentyl-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]propanenitrilephosphoricacid
26. Jakafi (tn)
27. Jakavi (tn)
28. Ruxolitinib Phosphate(incb018424)
29. Inc 424 Phosphate
30. Incb 018424 Phosphate
31. Incb-424
32. Schembl1369365
33. Chembl1795071
34. Amy5620
35. Dtxsid00911086
36. Ruxolitinib Phosphate (jan/usan)
37. Ruxolitinib Phosphate [mi]
38. Ruxolitinib Phosphate [jan]
39. Ex-a2660
40. Cs1956
41. Mfcd18452860
42. S5243
43. Akos024464417
44. Ruxolitinib (incb-18424) Phosphate
45. Ruxolitinib Phosphate [who-dd]
46. Bcp9000783
47. Ccg-268687
48. Cs-0326
49. 1h-pyrazole-1-propanenitrile, Beta-cyclopentyl-4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-,(betar)-, Phosphate (1:1)
50. Ac-30901
51. As-74723
52. Hy-50858
53. Ruxolitinib Phosphate [orange Book]
54. Ruxolitinib (as Phosphate) [ema Epar]
55. D09960
56. J-501793
57. Q27135517
58. (3r)-3-cyclopentyl-3-(4-{7h-pyrrolo[2,3-d]pyrimidin-4-yl}-1h-pyrazol-1-yl)propanenitrile; Phosphoric Acid
59. (3r)-3-cyclopentyl-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile;phosphoric Acid
60. (r)-3-(4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl)-3-cyclopentylpropanenitrilephosphate
61. 1h-pyrazole-1-propanenitrile,.beta.-cyclopentyl-4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-,(.beta.r)-,phosphate (1:1)
62. Phosphoric Acid--3-cyclopentyl-3-[4-(1h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]propanenitrile (1/1)
| Molecular Weight | 404.4 g/mol |
|---|---|
| Molecular Formula | C17H21N6O4P |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 404.13619017 g/mol |
| Monoisotopic Mass | 404.13619017 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 503 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Jakafi |
| PubMed Health | Ruxolitinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Ruxolitinib phosphate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 20mg base; eq 15mg base; eq 10mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Incyte |
| 2 of 2 | |
|---|---|
| Drug Name | Jakafi |
| PubMed Health | Ruxolitinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Ruxolitinib phosphate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 20mg base; eq 15mg base; eq 10mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Incyte |
* Myelofibrosis (MF):
Jakavi is indicated for the treatment of disease related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis.
* Polycythaemia vera (PV):
Jakavi is indicated for the treatment of adult patients with polycythaemia vera who are resistant to or intolerant of hydroxyurea.
* Graft versus host disease (GvHD):
Jakavi is indicated for the treatment of patients aged 12 years and older with acute graft versus host disease or chronic graft versus host disease who have inadequate response to corticosteroids or other systemic therapies (see section 5. 1).
Treatment of chronic Graft versus Host Disease (cGvHD)
Treatment of acute graft-versus-host disease (aGvHD)
Treatment of vitiligo
L01EJ01
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39763
Submission : 2024-03-27
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39494
Submission : 2024-03-29
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-11-16
Pay. Date : 2015-07-31
DMF Number : 29480
Submission : 2015-05-29
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38506
Submission : 2023-07-13
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2024-08-23
Pay. Date : 2024-06-28
DMF Number : 40066
Submission : 2024-07-24
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-12-16
Pay. Date : 2021-09-29
DMF Number : 32221
Submission : 2017-11-30
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39789
Submission : 2024-03-28
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39500
Submission : 2024-03-28
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38709
Submission : 2023-09-30
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
About the Company : Granules is a vertically integrated fast-growing Pharmaceutical company headquartered in Hyderabad with best-in-class facilities & a commitment to operational excellence, quality, ...
About the Company : Transo-Pharm, a fully licensed and certified distributor, specializes in pharmaceutical components for the health and veterinary industries. It offers support to clients throughout...
About the Company : Transo-Pharm, a fully licensed and certified distributor, specializes in pharmaceutical components for the health and veterinary industries. It offers support to clients throughout...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
About the Company : Established in 1990, Inabata France, a part of the Inabata Group, used to export chemical and pharmaceutical products to Japan. In 2006, it acquired Pharmasynthèse. Today, Inabata...
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients & provides custom-made solutions.
About the Company : Since its inception in 2003, Seqens has grown to become a global leader in pharmaceutical solutions and specialty ingredients. Seqens supports its customers in developing, scaling ...
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
About the Company : Faran Shimi Pharmaceutical Company, established in 2001 and affiliated with Golrang Pharmaceutical Investment Co, manufactures high-quality Active Pharmaceutical Ingredients (APIs)...
 Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
About the Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experience,the company has ...
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
About the Company : Aarti Pharmalab, earlier the pharma division of Aarti Industries, is a leading Indian manufacturer of APIs. It has dedicated facilities to manufacture HPAPIs, corticosteroids, cyto...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
1-(1-ethoxyethyl)-4-(4,4,5,5- tetramethyl-1,3,2-di...
CAS Number : 1029716-44-6
End Use API : Ruxolitinib Phosphate
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
CAS Number : 591769-05-0
End Use API : Ruxolitinib Phosphate
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
(4-(1-(2-Cyano-1- cyclopentylethyl)-1h-pyrazol-4-y...
CAS Number : 1236033-05-8
End Use API : Ruxolitinib Phosphate
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
1-(1-Ethoxyethyl)-4-(4,4,5,5-tetramethyl[1,3,2]dio...
CAS Number : 1029716-44-6
End Use API : Ruxolitinib Phosphate
About The Company : Allsino Pharmaceutical, established in May 2004, spans 97,350m² and specializes in the R&D and manufacturing of RSMs, GMP intermediates, and APIs. Our products...
4-Chloro-7H-pyrrolo-[2,3-d]pyrimidine
CAS Number : 3680-69-1
End Use API : Ruxolitinib Phosphate
About The Company : Allsino Pharmaceutical, established in May 2004, spans 97,350m² and specializes in the R&D and manufacturing of RSMs, GMP intermediates, and APIs. Our products...
4-Methyl-7-H-pyrrolo[2,3-d]pyrimidine
CAS Number : 945950-37-8
End Use API : Ruxolitinib Phosphate
About The Company : Allsino Pharmaceutical, established in May 2004, spans 97,350m² and specializes in the R&D and manufacturing of RSMs, GMP intermediates, and APIs. Our products...
3,7-Dihydro-4H-Pyrrolo[2,3-d] Pyrimidin-4-One
CAS Number : CAS-3680-71-5
End Use API : Ruxolitinib Phosphate
About The Company : Almelo are the industry leaders in manufacturing advanced intermediates, Active Pharmaceutical Ingredients (APIs) and specialty fine chemicals. With a diverse p...

4-hydroxypyrrolo[2,3-d]pyrimidine
CAS Number : 3680-71-5
End Use API : Ruxolitinib Phosphate
About The Company : Chengda Pharmaceutical Co., Ltd. was founded in 1999. It is located in the Hangzhou-Jiaxing-Huzhou plain, Jiashan Economic & Technological Development Zone (nat...

3- Cyclopentyl Acrilonitrile
CAS Number : 591769-05-0
End Use API : Ruxolitinib Phosphate
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

4- Chloro-7H-pyrrolo [2,3-d] pyrimidine
CAS Number : 3680-69-1
End Use API : Ruxolitinib Phosphate
About The Company : Established in 2011 and situated in Hangzhou, Zhejiang, China, Hangzhou Longshine Bio-Tech CO., Ltd is dedicated to providing services for pharmaceutical and ch...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24
Brand Name : JAKAVI
Patent Number : 2632466
Filing Date : 2006-12-12
Strength per Unit : 5 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24

Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24
Brand Name : JAKAVI
Patent Number : 2632466
Filing Date : 2006-12-12
Strength per Unit : 15 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24

Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24
Brand Name : JAKAVI
Patent Number : 2632466
Filing Date : 2006-12-12
Strength per Unit : 20 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24

Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24
Brand Name : JAKAVI
Patent Number : 2632466
Filing Date : 2006-12-12
Strength per Unit : 10 mg
Dosage Form : TABLET
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2026-12-12
Date Granted : 2013-09-24

Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09
Brand Name : JAKAVI
Patent Number : 2689663
Filing Date : 2008-06-12
Strength per Unit : 5 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09

Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09
Brand Name : JAKAVI
Patent Number : 2689663
Filing Date : 2008-06-12
Strength per Unit : 15 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09

Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09
Brand Name : JAKAVI
Patent Number : 2689663
Filing Date : 2008-06-12
Strength per Unit : 20 mg
Dosage Form : Tablet
Human Or VET : Human
Route of Administration : Oral
Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09

Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09
Brand Name : JAKAVI
Patent Number : 2689663
Filing Date : 2008-06-12
Strength per Unit : 10 mg
Dosage Form : TABLET
Human Or VET : Human
Route of Administration : ORAL
Patent Expiration Date : 2028-06-12
Date Granted : 2016-08-09

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?