
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
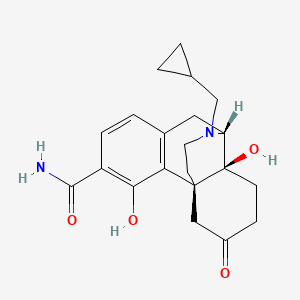

1. 3-carboxamido-4-hydroxynaltrexone
1. 852626-89-2
2. Alks 33
3. Alks-33
4. Samidorphan [usan]
5. Rdc-0313-00
6. Chembl426084
7. Rdc-0313
8. Samidorphan (usan)
9. 7w2581z5l8
10. Alks-5461 Component Samidorphan
11. 17-(cyclopropylmethyl)-4,14-dihydroxy-6-oxomorphinan-3-carboxamide
12. (1r,9r,10s)-17-(cyclopropylmethyl)-3,10-dihydroxy-13-oxo-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-triene-4-carboxamide
13. Morphinan-3-carboxamide, 17-(cyclopropylmethyl)-4,14-dihydroxy-6-oxo-
14. Chembl471243
15. (4br,8as,9r)-11-(cyclopropylmethyl)-4,8a-dihydroxy-6-oxo-6,7,8,8a,9,10-hexahydro-5h-9,4b-(epiminoethano)phenanthrene-3-carboxamide
16. Samidorphan [usan:inn]
17. Alks33
18. Unii-7w2581z5l8
19. Rdc 0313
20. Samidorphan [inn]
21. Samidorphan [who-dd]
22. Schembl2786239
23. Ccdc 710249, Hcl Salt
24. Gtpl10651
25. Chebi:177605
26. Dtxsid401030402
27. Lybalvi (olanzapine + Samidorphan)
28. Bdbm50165049
29. Bdbm50278265
30. Compound 5 [pmid:15808478]
31. Db12543
32. D10162
33. Q7409428
34. 17-cyclopropylmethyl-3,10-dihydroxy-13-oxo-(1r,9r,10s)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-triene-4-carboxamide
| Molecular Weight | 370.4 g/mol |
|---|---|
| Molecular Formula | C21H26N2O4 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 370.18925731 g/mol |
| Monoisotopic Mass | 370.18925731 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 665 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Samidorphan is indicated in combination with [olanzapine] for the treatment of bipolar I disorder, either as an adjunct to lithium or valproate or as monotherapy for the acute treatment of manic or mixed episodes or as maintenance therapy, and for the treatment of schizophrenia in adults.
Samidorphan, a novel opioid-system modulator, functions primarily as a -opioid receptor antagonist and as a /-opioid receptor partial agonist _in vitro_ with an overall profile consistent with a -opioid receptor antagonist _in vivo_ and is currently used to counteract [olanzapine]-induced adverse effects of weight gain and metabolic dysfunction. Samidorphin generally has a mild side effect profile. As an opioid antagonist, it can potentiate opioid withdrawal in dependent patients; it should not be administered within seven days from the last use of short-acting opioids and at least 14 days after cessation of long-acting opioids. Similarly, samidorphan use may lead to life-threatening opioid overdose, either in patients who attempt to overcome the samidorphan-induced opioid blockade or resume opioid use when therapy is interrupted or discontinued. Several other effects may be noted in combination with [olanzapine], including increased risk for potentially fatal cerebrovascular events in elderly patients with dementia-related psychosis, neuroleptic malignant syndrome, hyperprolactinemia, and tardive dyskinesia. [Olanzapine] is associated with an increased risk of metabolic dysfunction, including hyperglycemia, type II diabetes, dyslipidemia, and weight gain. Patients may be at increased risk for orthostatic hypotension and syncope, particularly in patients with known cardiovascular/cerebrovascular disease. Leukopenia, neutropenia, and agranulocytosis may occur and should be monitored for; patients with a history of seizure should also be monitored. This combination may exhibit anticholinergic (antimuscarinic) effects. It should be used with caution with other anticholinergic drugs and in patients with urinary retention, prostatic hypertrophy, constipation, paralytic ileus, or related conditions. Serious allergic reactions such as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) have been reported. Patients may be at increased risk of cognitive and motor impairment; caution while operating machinery is recommended.
Absorption
Samidorphan pharmacokinetics are linear over the range of clinically relevant concentrations, and steady-state kinetics are reached by seven days with once-daily oral administration. Upon reaching steady-state, with a once-daily dose of 10 mg samidorphan combined with 20 mg [olanzapine], samidorphan has a mean Cmax of 45.1 11.4 ng/mL and an AUC24h of 364 112 ng\*h/mL. Samidorphan has an absolute oral bioavailability of 69% and a Tmax of 1-2 hours. Samidorphan pharmacokinetics are not significantly impacted by food; following a high-fat meal, the Cmax was 0.85 (90% CI 0.76, 0.94) and the AUC 1.03 (90% CI 1.0, 1.05) that for the fasted state.
Route of Elimination
Samidorphan is primarily renally excreted, with 67% of unchanged parent and metabolites eliminated in urine and another 16% in feces.
Volume of Distribution
Samidorphan following a single 10 mg oral dose had an apparent volume of distribution between 336.59 75.42 and 557.6 120.51 L, depending on age, gender, and concomitant food consumption.
Clearance
Samidorphan has a mean clearance of 35-45 L/h.
Samidorphan is primarily metabolized by CYP3A4, with minor contributions from CYP3A5, CYP2C19, and CYP2C8. The main metabolism products are RDC-9986 (an N-dealkylated metabolite) and RDC-1066 (an N-oxide metabolite); although both metabolites have a nanomolar affinity for the -, -, and -opioid receptors, neither is thought to contribute to the pharmacological effects of samidorphan.
Samidorphan has a mean half-life of 7-11 hours.
Samidorphan is a novel [naltrexone] analogue containing a 3-carboxamido group that functions as an opioid receptor modulator, both _in vitro_ and _in vivo_. Numerous _in vitro_ studies have demonstrated that samidorphan binds with high affinity to the -, -, and -opioid receptors with Ki values of 0.052 0.0044, 0.23 0.018, and 2.7 0.36 nM, respectively. Samidorphan acts as an antagonist at the -opioid receptor when it signals through Gi proteins, a partial agonist when the receptor signals through GoA, GoB, and Gz proteins, and essentially lacks -arrestin-mediated signalling; samidorphan also acts as a partial agonist at both the - and -opioid receptors _in vitro_. In addition, both the major N-dealkylated and the major N-oxide human metabolites bind to the -, -, and -opioid receptors (Ki values of 0.26, 23, and 56, and 8, 110, and 280 nM, respectively); the former functions as a -opioid receptor agonist and the latter as an antagonist. Overall, samidorphan functions primarily as a -opioid antagonist _in vivo_. [Olanzapine] is an efficacious antipsychotic whose use is limited, in part, by known adverse effects mediated through metabolic dysfunction: hyperglycemia/diabetes mellitus, hyperlipidemia, and weight gain. The exact mechanisms behind this metabolic dysfunction are incompletely understood, but it is known that opioid signalling is involved in feeding and metabolism. Clinical studies have demonstrated that the addition of samidorphan to [olanzapine] helps mitigate its metabolic-related adverse effects; presumably, this is due to opioid receptor signalling, though the exact mechanism remains to be determined. The appropriateness of samidorphan in combination therapy is due in part to its relatively mild side effect profile and low abuse potential.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
98
PharmaCompass offers a list of Samidorphan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Samidorphan manufacturer or Samidorphan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Samidorphan manufacturer or Samidorphan supplier.
PharmaCompass also assists you with knowing the Samidorphan API Price utilized in the formulation of products. Samidorphan API Price is not always fixed or binding as the Samidorphan Price is obtained through a variety of data sources. The Samidorphan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Samidorphan L-Malate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Samidorphan L-Malate, including repackagers and relabelers. The FDA regulates Samidorphan L-Malate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Samidorphan L-Malate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Samidorphan L-Malate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Samidorphan L-Malate supplier is an individual or a company that provides Samidorphan L-Malate active pharmaceutical ingredient (API) or Samidorphan L-Malate finished formulations upon request. The Samidorphan L-Malate suppliers may include Samidorphan L-Malate API manufacturers, exporters, distributors and traders.
click here to find a list of Samidorphan L-Malate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Samidorphan L-Malate DMF (Drug Master File) is a document detailing the whole manufacturing process of Samidorphan L-Malate active pharmaceutical ingredient (API) in detail. Different forms of Samidorphan L-Malate DMFs exist exist since differing nations have different regulations, such as Samidorphan L-Malate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Samidorphan L-Malate DMF submitted to regulatory agencies in the US is known as a USDMF. Samidorphan L-Malate USDMF includes data on Samidorphan L-Malate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Samidorphan L-Malate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Samidorphan L-Malate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Samidorphan L-Malate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Samidorphan L-Malate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Samidorphan L-Malate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Samidorphan L-Malate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Samidorphan L-Malate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Samidorphan L-Malate suppliers with NDC on PharmaCompass.
Samidorphan L-Malate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Samidorphan L-Malate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Samidorphan L-Malate GMP manufacturer or Samidorphan L-Malate GMP API supplier for your needs.
A Samidorphan L-Malate CoA (Certificate of Analysis) is a formal document that attests to Samidorphan L-Malate's compliance with Samidorphan L-Malate specifications and serves as a tool for batch-level quality control.
Samidorphan L-Malate CoA mostly includes findings from lab analyses of a specific batch. For each Samidorphan L-Malate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Samidorphan L-Malate may be tested according to a variety of international standards, such as European Pharmacopoeia (Samidorphan L-Malate EP), Samidorphan L-Malate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Samidorphan L-Malate USP).

