
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
FDF
0
South Africa

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
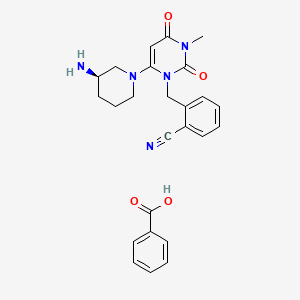

1. 2-((6-((3r)-3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)methyl) Benzonitrile
2. Alogliptin
3. Nesina
4. Syr 322
5. Syr-322
6. Syr322
1. 850649-62-6
2. Nesina
3. Syr 322
4. Syr-322
5. Alogliptin (benzoate)
6. (r)-2-((6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)methyl)benzonitrile Benzoate
7. 850649-62-6 (benzoate)
8. Alogliptin (as Benzoate)
9. Een99869sc
10. Chebi:72324
11. 2-((6-((3r)-3-amino-1-piperidinyl)-3,4-dihydro-3-methyl-2,4-dioxo-1(2h)- Pyrimidinyl)methyl)benzonitrile Monobenzoate
12. 2-[[6-[(3r)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile;benzoic Acid
13. 6-((3r)-3-aminopiperidin-1-yl)-1-(2-cyanobenzyl)-3-methylpyrimidin-2,4(1h,3h)-dione Monobenzoate
14. Benzonitrile, 2-((6-((3r)-3-amino-1-piperidinyl)-3,4-dihydro-3-methyl-2,4-dioxo-1(2h)-pyrimidinyl)methyl)-, Monobenzoate
15. Alogliptin, Benzoate
16. Alogliptin Benzoate [usan]
17. Unii-een99869sc
18. Mfcd09833195
19. Alogliptin Benzoate [usan:jan]
20. Vipidia (tn)
21. Nesina (tn)
22. Alogliptin Monobenzoate
23. Alogliptin(syr-322)
24. Schembl476231
25. Chembl227529
26. Alogliptin Benzoate [mi]
27. Alogliptin Benzoate (jan/usan)
28. Alogliptin Benzoate [jan]
29. Dtxsid20582095
30. Alogliptin Benzoate [vandf]
31. Alogliptin Benzoate [mart.]
32. Bcp08885
33. Hy-a0023
34. Ac-021
35. Alogliptin Benzoate [who-dd]
36. Akos015917686
37. Cs-0761
38. Alogliptin Benzoate [orange Book]
39. 2-[[6-[(3r)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2h)-pyrimidinyl]methyl]benzonitrile Benzoate
40. As-14073
41. Oseni Component Alogliptin Benzoate
42. Kazano Component Alogliptin Benzoate
43. Alogliptin Pound Syr-322 Pound(c)benzoate
44. Alogliptin Benzoate Component Of Oseni
45. Am20090704
46. Alogliptin Benzoate Component Of Kazano
47. D06553
48. Q27888443
49. Syr 322 Benzoate; Syr-322 Benzoate; Syr322 Benzoate
50. (3r)-1-[3-(2-cyanobenzyl)-1-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl]piperidin-3-aminium Benzoate
51. 2-((6-((3r)-3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)methyl)benzonitrile Monobenzoate
52. 2-({6-[(3r)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl}methyl)benzonitrile Benzoate
53. Benzoic Acid--2-({6-[(3r)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl}methyl)benzonitrile (1/1)
54. Benzonitrile, 2-[[6-[(3r)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2h)-pyrimidinyl]methyl]-, Benzoate (1:1)
| Molecular Weight | 461.5 g/mol |
|---|---|
| Molecular Formula | C25H27N5O4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 461.20630436 g/mol |
| Monoisotopic Mass | 461.20630436 g/mol |
| Topological Polar Surface Area | 131 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 726 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Nesina |
| PubMed Health | Alogliptin (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | NESINA tablets contain the active ingredient alogliptin, which is a selective, orally bioavailable inhibitor of the enzymatic activity of dipeptidyl peptidase-4 (DPP-4).Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-(... |
| Active Ingredient | Alogliptin benzoate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 12.5mg base; eq 6.25mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Nesina |
| PubMed Health | Alogliptin (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | NESINA tablets contain the active ingredient alogliptin, which is a selective, orally bioavailable inhibitor of the enzymatic activity of dipeptidyl peptidase-4 (DPP-4).Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-(... |
| Active Ingredient | Alogliptin benzoate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 12.5mg base; eq 6.25mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
Vipidia is indicated in adults aged 18 years and older with type 2 diabetes mellitus to improve glycaemic control in combination with other glucose lowering medicinal products including insulin, when these, together with diet and exercise, do not provide adequate glycaemic control (see sections 4. 4, 4. 5 and 5. 1 for available data on different combinations).
Dipeptidyl-Peptidase IV Inhibitors
Compounds that suppress the degradation of INCRETINS by blocking the action of DIPEPTIDYL-PEPTIDASE IV. This helps to correct the defective INSULIN and GLUCAGON secretion characteristic of TYPE 2 DIABETES MELLITUS by stimulating insulin secretion and suppressing glucagon release. (See all compounds classified as Dipeptidyl-Peptidase IV Inhibitors.)
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
Incretins
Peptides which stimulate INSULIN release from the PANCREATIC BETA CELLS following oral nutrient ingestion, or postprandially. (See all compounds classified as Incretins.)
A10BH04
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37908
Submission : 2022-12-30
Status : Active
Type : II

NDC Package Code : 42765-057
Start Marketing Date : 2023-01-24
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (10kg/10kg)
Marketing Category : BULK INGREDIENT
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 31293
Submission : 2016-12-30
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39984
Submission : 2024-07-23
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 28187
Submission : 2014-04-24
Status : Active
Type : II



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?