
Synopsis
0
VMF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
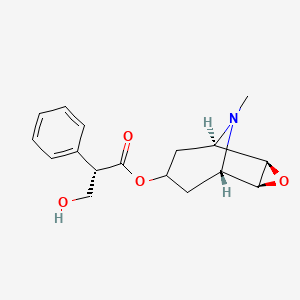

1. Boro Scopol
2. Boro-scopol
3. Hyoscine
4. Isopto Hyoscine
5. Kwells
6. Scoburen
7. Scopace
8. Scopoderm Tts
9. Scopolamine Cooper
10. Scopolamine Hydrobromide
11. Transderm Scop
12. Transderm V
13. Transderm-v
14. Travacalm Ho
15. Vorigeno
1. Hyoscine
2. (-)-hyoscine
3. 51-34-3
4. Scopine (-)-tropate
5. Scopine Tropate
6. (-)-scopolamine
7. 6,7-epoxytropine Tropate
8. Hyosol
9. Atrochin
10. Atroquin
11. Isopto Hyoscine
12. Skopolamin
13. Transderm-scop
14. L-scopolamine
15. Epoxytropine Tropate
16. Scopolamine Hydrobromide
17. 6-beta,7-beta-epoxy-3-alpha-tropanyl S-(-)-tropate
18. Beldavrin
19. Scopamin
20. Kwells
21. Alpha-(hydroxymethyl)benzeneacetic Acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl Ester
22. Hyosceine
23. Scop
24. Dl48g20x8x
25. Tropic Acid, Ester With Scopine
26. Chembl3084722
27. Chebi:16794
28. Euscopol
29. Isoscopil
30. Tranaxine
31. (1s,3s,5r,6r,7s)-6,7-epoxytropan-3-yl (2s)-3-hydroxy-2-phenylpropanoate
32. Hysco
33. Hyoscine Bromide
34. Scopace
35. Transderm Scop
36. Scopolamine Hcl
37. Boro-scopol
38. Hyoscine Hydrobromide
39. Scopolaminium Bromide
40. 1alphah,5alphah-tropan-3alpha-ol, 6beta,7beta-epoxy-, (-)-tropate (ester)
41. Scopolammonium Bromide
42. L-hyoscine Hydrobromide
43. See
44. (-)-scopolamine Bromide
45. L-scopolamine-hydrobromide
46. Scopolamine Bromide
47. (-)-hyoscine Hydrobromide
48. Scopoderm
49. Levo-duboisine
50. (+)-hyoscine
51. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0(2,4)]non-7-yl (2s)-3-hydroxy-2-phenylpropanoate
52. Hyoscyine Hydrobromide
53. Atroscine Hydrobromide
54. Hydroscine Hydrobromide
55. Hydrobromicum, Scopolaminum
56. Unii-dl48g20x8x
57. Tropane Alkaloid
58. Hsdb 4074
59. 6.beta.,7.beta.-epoxy-1.alpha.h,5.alpha.h-tropan-3.alpha.-ol (-)-tropate (ester)
60. Einecs 200-090-3
61. Nsc61806
62. S-(-)-tropate
63. Scopolamine [mi]
64. Prestwick3_000877
65. Hyoscine [mart.]
66. Scopolamine [hsdb]
67. Hyoscine [who-dd]
68. Ec 200-090-3
69. Scopolamine [vandf]
70. 6beta,7beta-epoxy-3alpha-tropanyl S-(-)-tropate
71. Schembl16226
72. Bspbio_000953
73. Gtpl330
74. 6beta,7beta-epoxy-1alpha,5alpha-tropan-3alpha-ol
75. Hyoscine [ep Impurity]
76. Bpbio1_001049
77. Chembl569713
78. Hyoscine [ep Monograph]
79. Chembl1906925
80. Dtxsid6023573
81. Schembl22393238
82. Chebi:93572
83. Scopolamine [orange Book]
84. Hms2090n13
85. Hms3886l22
86. Scopolamine [usp Impurity]
87. Hy-n0296
88. Lsm-4015
89. Ac-968
90. Bdbm50263508
91. S9326
92. Zinc13118910
93. Akos025402477
94. Tropic Acid, 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(sup 2,4))non-7-yl Ester
95. Zinc100037020
96. Zinc101147375
97. Ccg-267504
98. Cs-6609
99. Db00747
100. 3-oxa-9-azatricyclo(3.3.1.0(sup 2,4))nonan-7-ol, 9-methyl-, Tropate (ester)
101. 3-oxa-9-azatricyclo(3.3.1.o(sup 2,4))nonan-7-ol, 9-methyl-, Tropate (ester)
102. Smp1_000270
103. Ncgc00024357-04
104. Ncgc00024357-05
105. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0~2,4~]non-7-yl (2s)-3-hydroxy-2-phenylpropanoate
106. Ab00429689
107. Ab00429689-30
108. Ab00429689-31
109. Ab00429689_32
110. Atropine Sulfate Impurity F [ep Impurity]
111. Q337188
112. (methyl[?]yl) (2s)-3-hydroxy-2-phenyl-propanoate
113. Brd-k89923877-003-02-4
114. Q27165268
115. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl (2s)-3-hydroxy-2-phenylpropanoate
116. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0^{2,4}]nonan-7-yl (2s)-3-hydroxy-2-phenylpropanoate
117. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]non-7-yl (2r)-3-hydroxy-2-phenylpropanoate
118. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl (2s)-3-hydroxy-2-phenylpropanoate
119. (1r,2r,4s,5s,7s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl (s)-3-hydroxy-2-phenylpropanoate
120. [(1r,2r,4s,5s)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl] (2s)-3-hydroxy-2-phenylpropanoate
121. [7(s)-(1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.)]-.alpha.-(hydroxymethyl)benzeneacetic Acid 9-methyl-3-oxa-9-azatricyclo-[3.3.1.0^2,4]non-7-yl Ester
122. Benzeneacetic Acid, .alpha.(hydroxymethyl)-,(1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.)-9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, (.alpha.s)-
123. Benzeneacetic Acid, .alpha.-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, (7(s)-(1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.))-
124. Benzeneacetic Acid, Alpha-(hydroxymethyl)-, (1alpha,2beta,4beta,5alpha,7beta)-9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, (alphas)-
125. Benzeneacetic Acid, Alpha-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo(3.3.1.02,4)non-7-yl Ester, (7(s)-(1alpha,2beta,4beta,5alpha,7beta))-
1. Scopolamine Sulfate
2. Scopolamine Sulphate
| Molecular Weight | 303.35 g/mol |
|---|---|
| Molecular Formula | C17H21NO4 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 303.14705815 g/mol |
| Monoisotopic Mass | 303.14705815 g/mol |
| Topological Polar Surface Area | 62.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 418 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adjuvants, Anesthesia; Antiemetics; Muscarinic Antagonists; Mydriatics; Parasympatholytics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Although transdermal scopolamine has been shown to decrease basal acid output and inhibit betazole-, pentagastrin-, and peptone-stimulated gastric acid secretion in healthy individuals, it has not been determined whether transdermal scopolamine is effective in the adjunctive treatment of peptic ulcer disease. /Use is not currently included in the labeling approved by the US FDA/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1321
Transdermal scopolamine has shown minimal antiemetic activity against chemotherapy-induced vomiting. /Use is not currently included in the labeling approved by the US FDA/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1321
Scopolamine hydrobromide is used as a mydriatic and cycloplegic, especially when the patient is sensitive to atropine or when less prolonged cycloplegia is required. The effects of the drug appear more rapidly and have a shorter duration of action than those of atropine. Scopolamine hydrobromide is also used in the management of acute inflammatory conditions (i.e., iridocyclitis) of the iris and uveal tract. /Scopolamine hydrobromide/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2912
For more Therapeutic Uses (Complete) data for SCOPOLAMINE (10 total), please visit the HSDB record page.
The use of scopolamine to produce tranquilization and amnesia in a variety of circumstances, including labor, is declining and of questionable value. Given alone in the presence of pain or severe anxiety, scopolamine may induce outbursts of uncontrolled behavior.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 197
Scopolamine in therapeutic doses normally causes CNS depression manifested as drowsiness, amnesia, fatigue, and dreamless sleep, with a reduction in rapid eye movement (REM) sleep. It also causes euphoria and is therefore subject to some abuse. The depressant and amnesic effects formerly were sought when scopolamine was used as an adjunct to anesthetic agents or for preanesthetic medication. However, in the presence of severe pain, the same doses of scopolamine can occasionally cause excitement, restlessness, hallucinations, or delirium. These excitatory effects resemble those of toxic doses of atropine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 191-2
Scopolamine-induced inhibition of salivation occurs within 30 minutes or within 30 minutes to 1 hour and peaks within 1 or 1-2 hours after IM or oral administration, respectively; inhibition of salivation persists for up to 4-6 hours. Following IV administration of a 0.6-mg dose in one study, amnesia occurred within 10 minutes, peaked between 50-80 minutes, and persisted for at least 120 minutes after administration. Following IM administration of a 0.2-mg dose of scopolamine in one study, antiemetic effect occurred within 15-30 minutes and persisted for about 4 hours. Following IM administration of a 0.1- or 0.2-mg dose in another study, mydriasis persisted for up to 8 hours. The transdermal system is designed to provide an antiemetic effect with an onset of about 4 hours and with a duration of up to 72 hours after application.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
Small doses of ... scopolamine inhibit the activity of sweat glands innervated by sympathetic cholinergic fibers, and the skin becomes hot and dry. Sweating may be depressed enough to raise the body temperature, but only notably so after large doses or at high environmental temperatures.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 194
For more Drug Warnings (Complete) data for SCOPOLAMINE (21 total), please visit the HSDB record page.
Scopolamine is indicated in adult patients for the prevention of nausea and vomiting associated with motion sickness and for the prevention of postoperative nausea and vomiting (PONV) associated with anesthesia or opiate analgesia.
FDA Label
Scopolamine is an anticholinergic belladonna alkaloid that, through competitive inhibition of muscarinic receptors, affects parasympathetic nervous system function and acts on smooth muscles that respond to acetylcholine but lack cholinergic innervation. Formulated as a patch, scopolamine is released continuously over three days and remains detectable in urine over a period of 108 hours. Scopolamine is contraindicated in angle-closure glaucoma and should be used with caution in patients with open-angle glaucoma due to scopolamine's ability to increase intraocular pressure. Also, scopolamine exhibits several neuropsychiatric effects: exacerbated psychosis, seizures, seizure-like, and other psychiatric reactions, and cognitive impairment; scopolamine may impair the ability of patients to operate machinery or motor vehicles, play underwater sports, or perform any other potentially hazardous activity. Women with severe preeclampsia should avoid scopolamine. Patients with gastrointestinal or urinary disorders should be monitored frequently for impairments, and scopolamine should be discontinued if these develop. Scopolamine can cause blurred vision if applied directly to the eye, and the transdermal patch should be removed before an MRI procedure to avoid skin burns. Due to its gastrointestinal effects, scopolamine can interfere with gastric secretion testing and should be discontinued at least 10 days before performing the test. Finally, scopolamine may induce dependence and resulting withdrawal symptoms, such as nausea, dizziness, vomiting, gastrointestinal disturbances, sweating, headaches, bradycardia, hypotension, and various neuropsychiatric manifestations following treatment discontinuation; severe symptoms may require medical attention.
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Cholinergic Antagonists
Drugs that bind to but do not activate CHOLINERGIC RECEPTORS, thereby blocking the actions of ACETYLCHOLINE or cholinergic agonists. (See all compounds classified as Cholinergic Antagonists.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Mydriatics
Agents that dilate the pupil. They may be either sympathomimetics or parasympatholytics. (See all compounds classified as Mydriatics.)
A04AD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A04 - Antiemetics and antinauseants
A04A - Antiemetics and antinauseants
A04AD - Other antiemetics
A04AD01 - Scopolamine
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CM - Other hypnotics and sedatives
N05CM05 - Scopolamine
S - Sensory organs
S01 - Ophthalmologicals
S01F - Mydriatics and cycloplegics
S01FA - Anticholinergics
S01FA02 - Scopolamine
Absorption
The pharmacokinetics of scopolamine differ substantially between different dosage routes. Oral administration of 0.5 mg scopolamine in healthy volunteers produced a Cmax of 0.54 0.1 ng/mL, a tmax of 23.5 8.2 min, and an AUC of 50.8 1.76 ng\*min/mL; the absolute bioavailability is low at 13 1%, presumably because of first-pass metabolism. By comparison, IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a Cmax of 5.00 0.43 ng/mL, a tmax of 5.0 min, and an AUC of 369.4 2.2 ng\*min/mL. Other dose forms have also been tested. Subcutaneous administration of 0.4 mg scopolamine resulted in a Cmax of 3.27 ng/mL, a tmax of 14.6 min, and an AUC of 158.2 ng\*min/mL. Intramuscular administration of 0.5 scopolamine resulted in a Cmax of 0.96 0.17 ng/mL, a tmax of 18.5 4.7 min, and an AUC of 81.3 11.2 ng\*min/mL. Absorption following intranasal administration was found to be rapid, whereby 0.4 mg of scopolamine resulted in a Cmax of 1.68 0.23 ng/mL, a tmax of 2.2 3 min, and an AUC of 167 20 ng\*min/mL; intranasal scopolamine also had a higher bioavailability than that of oral scopolamine at 83 10%. Due to dose-dependent adverse effects, the transdermal patch was developed to obtain therapeutic plasma concentrations over a longer period of time. Following patch application, scopolamine becomes detectable within four hours and reaches a peak concentration (tmax) within 24 hours. The average plasma concentration is 87 pg/mL, and the total levels of free and conjugated scopolamine reach 354 pg/mL.
Route of Elimination
Following oral administration, approximately 2.6% of unchanged scopolamine is recovered in urine. Compared to this, using the transdermal patch system, less than 10% of the total dose, both as unchanged scopolamine and metabolites, is recovered in urine over 108 hours. Less than 5% of the total dose is recovered unchanged.
Volume of Distribution
The volume of distribution of scopolamine is not well characterized. IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a volume of distribution of 141.3 1.6 L.
Clearance
IV infusion of 0.5 mg scopolamine resulted in a clearance of 81.2 1.55 L/h, while subcutaneous administration resulted in a lower clearance of 0.14-0.17 L/h.
Scopolamine hydrobromide is rapidly absorbed following IM or subcutaneous injection. The drug is well absorbed from the GI tract, principally from the upper small intestine. Scopolamine also is well absorbed percutaneously. Following topical application behind the ear of a transdermal system, scopolamine is detected in plasma within 4 hours, with peak concentrations occurring within an average of 24 hours. In one study in healthy individuals, mean free and total (free plus conjugated) plasma scopolamine concentrations of 87 and 354 pg/mL, respectively, have been reported within 24 hours following topical application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours. /Scopolamine hydrobromide/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
Following oral administration of a 0.906-mg dose of scopolamine in one individual, a peak concentration of about 2 ng/mL was reached within 1 hour. Although the commercially available transdermal system contains 1.5 mg of scopolamine, the membrane-controlled diffusion system is designed to deliver approximately 1 mg of the drug to systemic circulation at an approximately constant rate over a 72-hour period. An initial priming dose of 0.14 mg of scopolamine is released from the adhesive layer of the system at a controlled, asymptotically declining rate over 6 hours; then, the remainder of the dose is released at an approximate rate of 5 ug/hour for the remaining 66-hour functional lifetime of the system. The manufacturer states that the initial priming dose saturates binding sites on the skin and rapidly brings the plasma concentration to steady-state. In a crossover study comparing urinary excretion rates of scopolamine during multiple 12-hour collection intervals in healthy individuals, there was no difference between the rates of excretion of drug during steady-state (24-72 hours) for constant-rate IV infusion (3.7-6 mcg/hour) and transdermal administration. The transdermal system appeared to deliver the drug to systemic circulation at the same rate as the constant-rate IV infusion; however, relatively long collection intervals (12 hours) make it difficult to interpret the data precisely. During the 12- to 24-hour period of administration and after 72 hours, the rate of excretion of scopolamine was higher with the transdermal system than with the constant-rate IV infusion.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
The distribution of scopolamine has not been fully characterized. The drug appears to be reversibly bound to plasma proteins. Scopolamine apparently crosses the blood-brain barrier since the drug causes CNS effects. The drug also reportedly crosses the placenta and is distributed into milk..
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
Although the metabolic and excretory fate of scopolamine has not been fully determined, the drug is thought to be almost completely metabolized (principally by conjugation) in the liver and excreted in urine. Following oral administration of a single dose of scopolamine in one study, only small amounts of the dose (about 4-5%) were excreted unchanged in urine within 50 hours; urinary clearance of unchanged drug was about 120 mL/minute. In another study, 3.4% or less than 1% of a single dose was excreted unchanged in urine within 72 hours following subcutaneous injection or oral administration of the drug, respectively. Following application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours in healthy individuals, the urinary excretion rate of free and total (free plus conjugated) scopolamine was about 0.7 and 3.8 ug/hour, respectively. Following removal of the transdermal system of scopolamine, depletion of scopolamine bound to skin receptors at the site of the application of the transdermal system results in a log-linear decrease in plasma scopolamine concentrations. Less than 10% of the total dose is excreted in urine as unchanged drug and its metabolites over 108 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
Little is known about the metabolism of scopolamine in humans, although many metabolites have been detected in animal studies. In general, scopolamine is primarily metabolized in the liver, and the primary metabolites are various glucuronide and sulphide conjugates. Although the enzymes responsible for scopolamine metabolism are unknown, _in vitro_ studies have demonstrated oxidative demethylation linked to CYP3A subfamily activity, and scopolamine pharmacokinetics were significantly altered by coadministration with grapefruit juice, suggesting that CYP3A4 is responsible for at least some of the oxidative demethylation.
Although the metabolic and excretory fate of scopolamine has not been fully determined, the drug is thought to be almost completely metabolized (principally by conjugation) in the liver and excreted in urine.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
The half-life of scopolamine differs depending on the route. Intravenous, oral, and intramuscular administration have similar half-lives of 68.7 1.0, 63.7 1.3, and 69.1 8/0 min, respectively. The half-life is greater with subcutaneous administration at 213 min. Following removal of the transdermal patch system, scopolamine plasma concentrations decrease in a log-linear fashion with a half-life of 9.5 hours.
Following application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours, the average elimination half-life of the drug was 9.5 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
[Acetylcholine] (ACh) is a neurotransmitter that can signal through ligand-gated cation channels (nicotinic receptors) and G-protein-coupled muscarinic receptors (mAChRs). ACh signalling via mAChRs located in the central nervous system (CNS) and periphery can regulate smooth muscle contraction, glandular secretions, heart rate, and various neurological phenomena such as learning and memory. mAChRs can be divided into five subtypes, M1-M5, expressed at various levels throughout the brain. Also, M2 receptors are found in the heart and M3 receptors in smooth muscles, mediating effects apart from the direct modulation of the parasympathetic nervous system. While M1, M3, and M5 mAChRs primarily couple to Gq proteins to activate phospholipase C, M2 and M4 mainly couple to Gi/o proteins to inhibit adenylyl cyclase and modulate cellular ion flow. This system, in part, helps to control physiological responses such as nausea and vomiting. Scopolamine acts as a non-selective competitive inhibitor of M1-M5 mAChRs, albeit with weaker M5 inhibition; as such, scopolamine is an anticholinergic with various dose-dependent therapeutic and adverse effects. The exact mechanism(s) of action of scopolamine remains poorly understood. Recent evidence suggests that M1 (and possibly M2) mAChR antagonism at interneurons acts through inhibition of downstream neurotransmitter release and subsequent pyramidal neuron activation to mediate neurological responses associated with stress and depression. Similar antagonism of M4 and M5 receptors is associated with potential therapeutic benefits in neurological conditions such as schizophrenia and substance abuse disorders. The significance of these observations to scopolamine's current therapeutic indications of preventing nausea and vomiting is unclear but is linked to its anticholinergic effect and ability to alter signalling through the CNS associated with vomiting.
Although other antimuscarinics have been used in the prevention of motion sickness, it appears that scopolamine is most effective. Scopolamine apparently corrects some central imbalance of acetylcholine and norepinephrine that may occur in patients with motion sickness. It has been suggested that antimuscarinics may block the transmission of cholinergic impulses from the vestibular nuclei to higher centers in the CNS and from the reticular formation to the vomiting center; these effects result in prevention of motion-induced nausea and vomiting.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1323
The sole active agent of Transderm Scoop is scopolamine, a belladonna alkaloid with well known pharmacological properties. It is an anticholinergic agent which acts: i) as a competitive inhibitor at postganglionic muscarinic receptor sites of the parasympathetic nervous system, and ii) on smooth muscles that respond to acetylcholine but lack cholinergic innervation. It has been suggested that scopolamine acts in the central nervous system (CNS) by blocking cholinergic transmission from the vestibular nuclei to higher centers in the CNS and from the reticular formation to the vomiting center.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 2192

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
64
PharmaCompass offers a list of Scopolamine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Scopolamine manufacturer or Scopolamine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Scopolamine manufacturer or Scopolamine supplier.
PharmaCompass also assists you with knowing the Scopolamine API Price utilized in the formulation of products. Scopolamine API Price is not always fixed or binding as the Scopolamine Price is obtained through a variety of data sources. The Scopolamine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Scopolamine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Scopolamine, including repackagers and relabelers. The FDA regulates Scopolamine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Scopolamine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Scopolamine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Scopolamine supplier is an individual or a company that provides Scopolamine active pharmaceutical ingredient (API) or Scopolamine finished formulations upon request. The Scopolamine suppliers may include Scopolamine API manufacturers, exporters, distributors and traders.
click here to find a list of Scopolamine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Scopolamine DMF (Drug Master File) is a document detailing the whole manufacturing process of Scopolamine active pharmaceutical ingredient (API) in detail. Different forms of Scopolamine DMFs exist exist since differing nations have different regulations, such as Scopolamine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Scopolamine DMF submitted to regulatory agencies in the US is known as a USDMF. Scopolamine USDMF includes data on Scopolamine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Scopolamine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Scopolamine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Scopolamine Drug Master File in Japan (Scopolamine JDMF) empowers Scopolamine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Scopolamine JDMF during the approval evaluation for pharmaceutical products. At the time of Scopolamine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Scopolamine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Scopolamine Drug Master File in Korea (Scopolamine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Scopolamine. The MFDS reviews the Scopolamine KDMF as part of the drug registration process and uses the information provided in the Scopolamine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Scopolamine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Scopolamine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Scopolamine suppliers with KDMF on PharmaCompass.
A Scopolamine CEP of the European Pharmacopoeia monograph is often referred to as a Scopolamine Certificate of Suitability (COS). The purpose of a Scopolamine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Scopolamine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Scopolamine to their clients by showing that a Scopolamine CEP has been issued for it. The manufacturer submits a Scopolamine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Scopolamine CEP holder for the record. Additionally, the data presented in the Scopolamine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Scopolamine DMF.
A Scopolamine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Scopolamine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Scopolamine suppliers with CEP (COS) on PharmaCompass.
A Scopolamine written confirmation (Scopolamine WC) is an official document issued by a regulatory agency to a Scopolamine manufacturer, verifying that the manufacturing facility of a Scopolamine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Scopolamine APIs or Scopolamine finished pharmaceutical products to another nation, regulatory agencies frequently require a Scopolamine WC (written confirmation) as part of the regulatory process.
click here to find a list of Scopolamine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Scopolamine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Scopolamine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Scopolamine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Scopolamine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Scopolamine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Scopolamine suppliers with NDC on PharmaCompass.
Scopolamine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Scopolamine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Scopolamine GMP manufacturer or Scopolamine GMP API supplier for your needs.
A Scopolamine CoA (Certificate of Analysis) is a formal document that attests to Scopolamine's compliance with Scopolamine specifications and serves as a tool for batch-level quality control.
Scopolamine CoA mostly includes findings from lab analyses of a specific batch. For each Scopolamine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Scopolamine may be tested according to a variety of international standards, such as European Pharmacopoeia (Scopolamine EP), Scopolamine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Scopolamine USP).
