
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
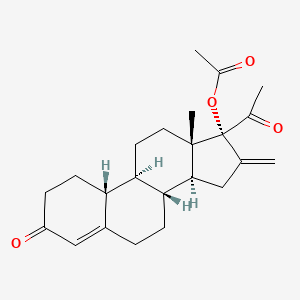

1. 16-methylene-17alpha-acetoxy-19-nor-4-pregnen-3,20-dione
2. St 1435
3. St-1435
1. Nestoron
2. 7759-35-5
3. Elcometrine
4. Segesterone Acetate
5. St-1435
6. Segesterone Acetate [usan]
7. 17-hydroxy-16-methylene-19-norpregn-4-ene-3,20-dione Acetate
8. 9amx4q13cc
9. 16-methylene-17-alpha-acetoxy-19-nor-4-pregnene-3,20-dione
10. 19-norpregn-4-ene-3,20-dione, 17-(acetyloxy)-16-methylene-
11. Segesterone Acetate (usan)
12. St 1435
13. (8r,9s,10r,13s,14s,17r)-17-acetyl-13-methyl-16-methylene-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Acetate
14. Unii-9amx4q13cc
15. Segesterone-acetate
16. Nestorone (tn)
17. [(8r,9s,10r,13s,14s,17r)-17-acetyl-13-methyl-16-methylidene-3-oxo-2,6,7,8,9,10,11,12,14,15-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Acetate
18. 16-methylene-17alpha-acetoxy-19-nor-4-pregnen-3,20-dione
19. Elcometrine [mi]
20. Schembl1261001
21. Chembl3707377
22. Elcometrine;nestorone;st-1435
23. Nestorone, >=97% (hplc)
24. Dtxsid70998804
25. Chebi:135563
26. 16-methylene-17-hydroxy-19-norpregn-4-ene-3,20-dione Acetate
27. Bcp12737
28. Zinc5167230
29. 19-norpregn-4-ene-3,20-dione, 17-hydroxy-16-methylene-, Acetate
30. Segesterone Acetate [who-dd]
31. Akos025402243
32. Ac-6844
33. Cs-0411
34. Db14583
35. Ncgc00487114-02
36. Segesterone Acetate [orange Book]
37. Hy-13071
38. Annovera Component Segesterone Acetate
39. D10986
40. E89278
41. Segesterone Acetate Component Of Annovera
42. 759n355
43. A914499
44. Elcometrine;nestorone;st-1435;st 1435;st1435
45. Q1978481
46. 1-tert-butoxycarbonylamino-cyclopent-3-enecarboxylicacid
47. 16-methylene-17.alpha.-acetoxy-19-nor-pregn-4-ene-3,20-dione
48. 17-(acetyoxy)-16- Methylene -19-nonpregn-4-ene-3,20-dione
| Molecular Weight | 370.5 g/mol |
|---|---|
| Molecular Formula | C23H30O4 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 370.21440943 g/mol |
| Monoisotopic Mass | 370.21440943 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 762 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Segesterone acetate in combination with ethinyl estradiol is indicated for use by females of reproductive potential to prevent pregnancy as a combination hormonal contraceptive (CHC). It induces contraception for thirteen 28-day cycles (1 year) following vaginal administration. The vaginal system must remain in place continuously for 3 weeks (21 days) followed by a 1-week (7-day) vaginal system-free interval. The use in females with a body mass index of >29 kg/m^2 has not been adequately evaluated.
FDA Label
Segesterone acetate suppresses ovulation. In a Phase I randomized, placebo-controlled, randomized crossover study involving healthy adult female subjects, there was no clinically significant QTc interval prolongation following a single intravenous bolus dose of segesterone acetate. Segesterone acetate shows no androgenic, anabolic, or estrogenic activity. It also did not show uterotropic activity in ovariectomized rats. In the endometrial transformation test to assess the progestational activity, dose-dependent increases in both uterine weight was observed following subcutaneous administration of segesterone acetate.
Contraceptive Agents, Female
Chemical substances or agents with contraceptive activity in females. Use for female contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Female.)
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
Absorption
Contraceptive vaginal rings provided sustained release of contraceptive levels of segesterone acetate over 90 days in a pharmacokinetic study of healthy women. Following vaginal administration for up to 13 cycles, segesterone acetate was absorbed into systemic administration and reached the peak plasma concentration in 2 hours in Cycle 1, Cycle 3, and Cycle 13. Concentrations declined after time to reach plasma concentration (Tmax) and became more constant after 96 hours post-dose.Over subsequent cycles of use, the peak serum concentrations of segesterone acetate decreased. In Cycle 1, 3 and 13, the peak plasma concentrations were 1147, 363, and 294 pg/mL.
Route of Elimination
In a pharmacokinetic study, approximately 81.4% and 7.62% of the subcutaneously-administered dose in rats was excreted via feces and urine, respectively.
Volume of Distribution
The volume of distribution of segesterone acetate is 19.6 L/kg.
Clearance
No pharmacokinetic data available.
Segesterone acetate undergoes rapid metabolism and inactivation in the liver. Based on the findings _in vitro_, the major oxidative metabolites in the serum include 5-dihydro- and 17-hydroxy-5-dihydro metabolites constitute about 50% of exposure relative to segesterone acetate. The metabolites are not pharmacologically active with EC50 to progesterone receptor 10-fold higher than that of the parent compound. It was shown that 3, 5-tetrahydrosegesterone acetate acts as an activator at the GABA-A receptors in the brain.
The mean (SD) half life of segesterone acetate is 4.5 (3.4) hours.
Segesterone acetate selectively binds to the progesterone receptor (PR), a transcription factor belonging to the nuclear receptor superfamily, where it acts as an agonist and transactivator. According to the findings from docking experiments, it adopts the same docking position within the PR ligand-binding domain (LBD) as progesterone but due to additional stabilizing contacts between 17-acetoxy and 16-methylene groups and PR LBD, segesterone acetate display higher potency than progesterone. As with other progestins, segesterone acetate prevents ovulation by blocking the midcycle surge in luteinizing hormone (LH) secretion, thereby inhibiting the development of ovarian follicles. When used in combination with segesterone acetate, ethinyl estradiol potentiates the antigonadotropic of the progestin and prevents irregular shedding of the endometrium. Segesterone acetate lacks androgenic activity, and displayed binding affinity to androgen receptors that was 500- to 600-fold less than that of testosterone. It does not display binding affinity toward estrogen receptors. When the relative binding affinities of segesterone acetate to human steroid receptors were investigated _in vitro_, it was demonstrated that segesterone acetate binds to the glucocorticoid receptor. However, segesterone acetate did not exert any glucocorticoid activity in the _in vivo_ assays showing no increase in liver glycogen and tyrosine transaminase TAT.
Global Sales Information

Market Place

Reply
02 Sep 2022
Reply
14 Sep 2017
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
18
PharmaCompass offers a list of Segesterone Acetate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Segesterone Acetate manufacturer or Segesterone Acetate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Segesterone Acetate manufacturer or Segesterone Acetate supplier.
PharmaCompass also assists you with knowing the Segesterone Acetate API Price utilized in the formulation of products. Segesterone Acetate API Price is not always fixed or binding as the Segesterone Acetate Price is obtained through a variety of data sources. The Segesterone Acetate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Segesterone Acetate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Segesterone Acetate, including repackagers and relabelers. The FDA regulates Segesterone Acetate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Segesterone Acetate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Segesterone Acetate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Segesterone Acetate supplier is an individual or a company that provides Segesterone Acetate active pharmaceutical ingredient (API) or Segesterone Acetate finished formulations upon request. The Segesterone Acetate suppliers may include Segesterone Acetate API manufacturers, exporters, distributors and traders.
click here to find a list of Segesterone Acetate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Segesterone Acetate DMF (Drug Master File) is a document detailing the whole manufacturing process of Segesterone Acetate active pharmaceutical ingredient (API) in detail. Different forms of Segesterone Acetate DMFs exist exist since differing nations have different regulations, such as Segesterone Acetate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Segesterone Acetate DMF submitted to regulatory agencies in the US is known as a USDMF. Segesterone Acetate USDMF includes data on Segesterone Acetate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Segesterone Acetate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Segesterone Acetate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Segesterone Acetate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Segesterone Acetate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Segesterone Acetate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Segesterone Acetate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Segesterone Acetate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Segesterone Acetate suppliers with NDC on PharmaCompass.
Segesterone Acetate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Segesterone Acetate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Segesterone Acetate GMP manufacturer or Segesterone Acetate GMP API supplier for your needs.
A Segesterone Acetate CoA (Certificate of Analysis) is a formal document that attests to Segesterone Acetate's compliance with Segesterone Acetate specifications and serves as a tool for batch-level quality control.
Segesterone Acetate CoA mostly includes findings from lab analyses of a specific batch. For each Segesterone Acetate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Segesterone Acetate may be tested according to a variety of international standards, such as European Pharmacopoeia (Segesterone Acetate EP), Segesterone Acetate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Segesterone Acetate USP).
