
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
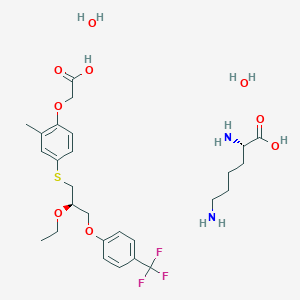

1. Mbx-8025 Lysine
2. Seladelpar Lysine Dihydrate
3. Mbx-8025 Lysine Dihydrate
4. 928821-40-3
5. Seladelpar Lysine [usan]
6. Mbx-8025 Lysine Salt, Dihydrate
7. N1429130kr
8. Seladelpar (lysine Dihydrate)
9. Unii-n1429130kr
10. Seladelpar Lysine (usan)
11. Chembl3989960
12. Wtkswpygzdcunq-jzxfcxspsa-n
13. Hy-19522c
14. Akos040747473
15. Cs-1068545
16. D11257
17. Q27284370
18. (2s)-2,6-diaminohexanoic Acid;2-[4-[(2r)-2-ethoxy-3-[4-(trifluoromethyl)phenoxy]propyl]sulfanyl-2-methylphenoxy]acetic Acid;dihydrate
19. (r)-2-(4-((2-ethoxy-3-(4-(trifluoromethyl)phenoxy)propyl)thio)-2-methylphenoxy)acetic Acid Compound With (s)-2,6-diaminohexanoic Acid Dihydrate
20. L-lysine (4-(((2r)-2-ethoxy-3-(4-(trifluoromethyl)phenoxy)propyl)sulfanyl)-2-methylphenoxy)acetate Dihydrate
21. L-lysine, 2-(4-(((2r)-2-ethoxy-3-(4-(trifluoromethyl)phenoxy)propyl)thio)-2-methylphenoxy)acetate, Hydrate (1:1:2)
1. 851528-79-5
2. Mbx-8025
3. Mbx-8025 (seladelpar)
4. Seladelpar
| Molecular Weight | 626.7 g/mol |
|---|---|
| Molecular Formula | C27H41F3N2O9S |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 16 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 182 |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 617 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |


NDC Package Code : 48957-0106
Start Marketing Date : 2024-08-14
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
