
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
Canada

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
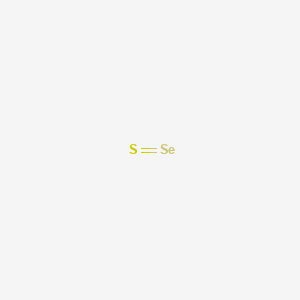
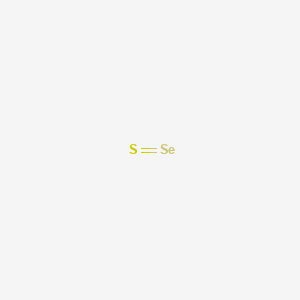
1. Selenium Sulphide
2. 7446-34-6
3. Selenium Sulfide (ses)
4. Selensulfid
5. Selensulfid [german]
6. Ccris 554
7. Hsdb 679
8. Nci-c50033
9. Unii-j90wfr7zff
10. Selenium Sulfide Red Powder
11. Selenosulfide
12. Abbotselsun
13. Caspiselenium
14. Selensulfur
15. Selenenyl Sulfide
16. Selenium Sulfide Usp
17. Ses2
18. Dtxsid9021265
19. 446s346
20. Q27430357
1. Selsun
2. Selenium Disulfide
3. Lenium
| Molecular Weight | 111.04 g/mol |
|---|---|
| Molecular Formula | SSe |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 111.88859 g/mol |
| Monoisotopic Mass | 111.88859 g/mol |
| Topological Polar Surface Area | 32.1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Selenium sulfide |
| PubMed Health | Selenium Sulfide (On the skin) |
| Drug Classes | Antiseborrheic, Dermatological Agent |
| Drug Label | Each mL contains 23 mg of selenium sulfide in a vehicle consisting of: ammonium lauryl sulfate, caprylic capric triglyceride, chromium oxide green, citric acid, cocamidopropyl betaine, D&C yellow #8, diazolidinyl urea, disodium EDTA, FD&C red #40, |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Wockhardt; Perrigo New York |
| 2 of 4 | |
|---|---|
| Drug Name | Selsun |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Chattem |
| 3 of 4 | |
|---|---|
| Drug Name | Selenium sulfide |
| PubMed Health | Selenium Sulfide (On the skin) |
| Drug Classes | Antiseborrheic, Dermatological Agent |
| Drug Label | Each mL contains 23 mg of selenium sulfide in a vehicle consisting of: ammonium lauryl sulfate, caprylic capric triglyceride, chromium oxide green, citric acid, cocamidopropyl betaine, D&C yellow #8, diazolidinyl urea, disodium EDTA, FD&C red #40, |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Wockhardt; Perrigo New York |
| 4 of 4 | |
|---|---|
| Drug Name | Selsun |
| Active Ingredient | Selenium sulfide |
| Dosage Form | Lotion/shampoo |
| Route | Topical |
| Strength | 2.5% |
| Market Status | Prescription |
| Company | Chattem |
/Experimental Therapy/ ... This randomized, double-blind, placebo-controlled intervention study included 725 institutionalized elderly patients (>65 years) from 25 geriatric centers in France. Patients received an oral daily supplement of nutritional doses of trace elements (zinc and selenium sulfide) or vitamins (beta carotene, ascorbic acid, and vitamin E) or a placebo within a 2 x 2 factorial design for 2 years. ... Correction of specific nutrient deficiencies was observed after 6 months of supplementation and was maintained for the first year, during which there was no effect of any treatment on delayed-type hypersensitivity skin response. Antibody titers after influenza vaccine were higher in groups that received trace elements alone or associated with vitamins, whereas the vitamin group had significantly lower antibody titers (P<.05). The number of patients without respiratory tract infections during the study was higher in groups that received trace elements (P = .06). Supplementation with neither trace elements nor vitamins significantly reduced the incidence of urogenital infections. Survival analysis for the 2 years did not show any differences between the 4 groups. CONCLUSIONS: Low-dose supplementation of zinc and selenium provides significant improvement in elderly patients by increasing the humoral response after vaccination and could have considerable public health importance by reducing morbidity from respiratory tract infections.
PMID:10218756 Girodon F et al; Arch Intern Med 159 (7): 748-54 (1999)
/Experimental Therapy/ Forty children aged 1-11 years with clinically diagnosed tinea capitis were randomized to receive selenium sulfide shampoo 1% or ciclopirox shampoo 1% twice a week as adjuncts to an 8-week course of ultramicronized griseofulvin dosed at 10-12 mg/kg/day. At weeks 2, 4, and 8, subjects returned to the clinic for evaluation and scalp cultures. Subjects then returned for follow-up visits 4 weeks after completing treatment. Overall, by 8 weeks, 30 of 33 (90.9%) treated children demonstrated mycological cure. Selenium sulfide shampoo 1% and ciclopirox shampoo 1% were equally effective as adjunctive treatments for tinea capitis in children in our study.
PMID:20735804 Chen C et al; Pediatr Dermatol 27 (5): 459-62 (2010)
Two cases of new chemicals causing yellow hair shaft discoloration are reported. The chemicals include selenium sulfide 2.5% shampoo and dihydroxyacetone.
PMID:18664164 Prevost N, English JC III; J Drugs Dermatol 7 (77): 689-91 (2008)
... Ingestion is hazardous. If swallowed, avoid oils or alcohol which may promote absorption.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-129
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE13 - Selenium sulfide
The percutaneous absorption of selenium sulfide was ... studied in /a/ groups who applied the drug to the entire skin for five minutes for eighteen days. Fluorimetric analysis of urinary samples collected on the third and thirteenth days of treatment revealed no significant increase in the excretion of selenium as compared to pretreatment levels. Systemic toxicity was not observed in any of the patients treated. The results suggest that the selenium sulfide is absorbed poorly from the skin...
PMID:988451 Costa Martins JE et al; Med Cutan Ibero Lat Am 4 (2): 137-41 (1976)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
41
PharmaCompass offers a list of Selenium Sulfide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Selenium Sulfide manufacturer or Selenium Sulfide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Selenium Sulfide manufacturer or Selenium Sulfide supplier.
PharmaCompass also assists you with knowing the Selenium Sulfide API Price utilized in the formulation of products. Selenium Sulfide API Price is not always fixed or binding as the Selenium Sulfide Price is obtained through a variety of data sources. The Selenium Sulfide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Selenium Sulfide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Selenium Sulfide, including repackagers and relabelers. The FDA regulates Selenium Sulfide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Selenium Sulfide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Selenium Sulfide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Selenium Sulfide supplier is an individual or a company that provides Selenium Sulfide active pharmaceutical ingredient (API) or Selenium Sulfide finished formulations upon request. The Selenium Sulfide suppliers may include Selenium Sulfide API manufacturers, exporters, distributors and traders.
click here to find a list of Selenium Sulfide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Selenium Sulfide DMF (Drug Master File) is a document detailing the whole manufacturing process of Selenium Sulfide active pharmaceutical ingredient (API) in detail. Different forms of Selenium Sulfide DMFs exist exist since differing nations have different regulations, such as Selenium Sulfide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Selenium Sulfide DMF submitted to regulatory agencies in the US is known as a USDMF. Selenium Sulfide USDMF includes data on Selenium Sulfide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Selenium Sulfide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Selenium Sulfide suppliers with USDMF on PharmaCompass.
A Selenium Sulfide written confirmation (Selenium Sulfide WC) is an official document issued by a regulatory agency to a Selenium Sulfide manufacturer, verifying that the manufacturing facility of a Selenium Sulfide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Selenium Sulfide APIs or Selenium Sulfide finished pharmaceutical products to another nation, regulatory agencies frequently require a Selenium Sulfide WC (written confirmation) as part of the regulatory process.
click here to find a list of Selenium Sulfide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Selenium Sulfide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Selenium Sulfide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Selenium Sulfide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Selenium Sulfide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Selenium Sulfide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Selenium Sulfide suppliers with NDC on PharmaCompass.
Selenium Sulfide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Selenium Sulfide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Selenium Sulfide GMP manufacturer or Selenium Sulfide GMP API supplier for your needs.
A Selenium Sulfide CoA (Certificate of Analysis) is a formal document that attests to Selenium Sulfide's compliance with Selenium Sulfide specifications and serves as a tool for batch-level quality control.
Selenium Sulfide CoA mostly includes findings from lab analyses of a specific batch. For each Selenium Sulfide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Selenium Sulfide may be tested according to a variety of international standards, such as European Pharmacopoeia (Selenium Sulfide EP), Selenium Sulfide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Selenium Sulfide USP).
