
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
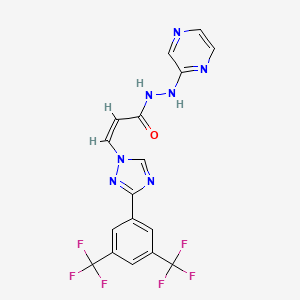

1. Kpt-330
2. Xpovio
1. Kpt-330
2. 1393477-72-9
3. Xpovio
4. Selinexor (kpt-330)
5. Selinexor Free Base
6. (z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1h-1,2,4-triazol-1-yl)-n'-(pyrazin-2-yl)acrylohydrazide
7. Kpt 330
8. 31tz62fo8f
9. 2-propenoic Acid, 3-(3-(3,5-bis(trifluoromethyl)phenyl)-1h-1,2,4-triazol-1-yl)-, 2-(2-pyrazinyl)hydrazide, (2z)-
10. (z)-3-[3-[3,5-bis(trifluoromethyl)phenyl]-1,2,4-triazol-1-yl]-n'-pyrazin-2-ylprop-2-enehydrazide
11. 1621865-82-4
12. 2-propenoic Acid, 3-[3-[3,5-bis(trifluoromethyl)phenyl]-1h-1,2,4-triazol-1-yl]-, 2-(2-pyrazinyl)hydrazide, (2z)-
13. Selinexor [usan:inn]
14. Unii-31tz62fo8f
15. Nexpovio
16. Selinexor [inn]
17. Kpt330;selinexor
18. Xpovio (tn)
19. Kpt-330(selinexor)
20. Selinexor [mi]
21. Selinexor (usan/inn)
22. Selinexor [usan]
23. Selinexor [who-dd]
24. Selinexor [orange Book]
25. Chembl3545185
26. Schembl14678327
27. Gtpl10036
28. Ex-a870
29. Dtxsid801026013
30. Bdbm50527778
31. Mfcd27987944
32. Nsc780203
33. Nsc781780
34. Zinc96170454
35. Ccg-269161
36. Db11942
37. Nsc-780203
38. Nsc-781780
39. Ncgc00386310-01
40. Ncgc00386310-03
41. Ac-33645
42. Bs-15022
43. Compound 70 [wo2013019561a1]
44. N-hydroxy-n'-(2-phenylethyl)isophthalamide
45. S7252
46. Sw219336-1
47. D11222
48. A857179
49. J-690156
50. Q27256082
51. (2z)-2-(2-pyrazinyl)hydrazide-3-[3-[3,5-bis(trifluoromethyl)phenyl]-1h-1,2,4-triazol-1-yl]-2-propenoic Acid
52. Selinexor;(z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1h-1,2,4-triazol-1-yl)-n'-(pyrazin-2-yl)acrylohydrazide
| Molecular Weight | 443.3 g/mol |
|---|---|
| Molecular Formula | C17H11F6N7O |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 5 |
| Exact Mass | 443.09292697 g/mol |
| Monoisotopic Mass | 443.09292697 g/mol |
| Topological Polar Surface Area | 97.6 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 621 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Selinexor is indicated for the treatment of relapsed or refractory multiple myeloma in combination with dexamethasone. Patients must have received at least 4 prior therapies and have disease which is refractory to least two proteasome inhibitors, at least two immunomodulatory agents, and an antiCD38 monoclonal antibody.
FDA Label
NEXPOVIO is indicated
- in combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy.
- in combination with dexamethasone for the treatment of multiple myeloma in adult patients who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.
Selinexor causes cell cycle arrest and apoptosis in cancer cells.
L01XX66
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX66 - Selinexor
Absorption
A single 80 mg dose of selinexor produces a mean Cmax of 680 ng/mL and a mean AUC of 5386 ng*h/mL. This relationship is dose proportion over the range of 3-85 mg/m2 which encompasses the range of 0.06-1.8 times the approved dosage. The official FDA labeling reports the Tmax as 4 hours but phase 1 studies have found a range of 2-4 hours. Administering selinexor with food, either a high or low fat meal, results in an increase in the AUC of approximately 15-20% but this is not expected to be clinically significant.
Volume of Distribution
The mean apparent volume of distribution is 125 L. A phase 1 study reported mean apparent volumes of distribution ranging from 1.9-2.9 L/kg in their investigation of food and formulation effects.
Clearance
Selinexor has a mean apparent clearance of 17.9 L/h.
Selinexor is known to be metabolized through CYP3A4, UDPglucuronosyltransferases, and glutathione S-transferases although the metabolite profile has yet to be characterized in published literature. The primary metabolites found in urine and plasma are glucuronide conjugates.
Selinexor has a mean half-life of elimination of 6-8 hours.
Selinexor binds to and inhibits exportin-1 (XPO1). XPO1 is a nuclear exporter protein which contains a pocket to which nuclear proteins can bind. When complexed with these proteins and Ran, activated through guanosine triphosphate (GTP) binding, the XPO1-protein-Ran-GTP complex is able to exit the nucleus through a nuclear pore. Once outside, GTP is hydrolyzed and the complex dissociates. The inhibition of this process in cancer cells allows the targets of XPO1, many of which are tumor suppressors, to collect in the nucleus and result in increased transcription of tumor suppressor genes. Tumor suppressor proteins known to be affected by XPO1 inhibition include p53, p73, adenomatous polyposis coli, retinoblastoma, forkhead box protein O, breast cancer 1, nucleophosmin, and merlin. Regulators of cell cycle progression are also affected, namely p21, p27, galectin-3, and Tob. Inhibitor of NFB also collects in the nucleus as a result leading to reduced activity of NFB, a known contributor to cancer. XPO1 participates in the formation of a complex with eukaryotic initiation factor 4E and contributes to the transport of messenger RNA for several oncegenes including cell cycle promotors, cyclin D1, cyclin E, and CDK2/4/6, as well as antiapoptotic proteins, Mcl-1 and Bcl-xL. These wide ranging changes in protein expression and gene transcription culminate in cell cycle arrest and the promotion of apoptosis in cancer cells.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
80
PharmaCompass offers a list of Selinexor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Selinexor manufacturer or Selinexor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Selinexor manufacturer or Selinexor supplier.
PharmaCompass also assists you with knowing the Selinexor API Price utilized in the formulation of products. Selinexor API Price is not always fixed or binding as the Selinexor Price is obtained through a variety of data sources. The Selinexor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Selinexor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Selinexor, including repackagers and relabelers. The FDA regulates Selinexor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Selinexor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Selinexor manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Selinexor supplier is an individual or a company that provides Selinexor active pharmaceutical ingredient (API) or Selinexor finished formulations upon request. The Selinexor suppliers may include Selinexor API manufacturers, exporters, distributors and traders.
click here to find a list of Selinexor suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Selinexor Drug Master File in Korea (Selinexor KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Selinexor. The MFDS reviews the Selinexor KDMF as part of the drug registration process and uses the information provided in the Selinexor KDMF to evaluate the safety and efficacy of the drug.
After submitting a Selinexor KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Selinexor API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Selinexor suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Selinexor as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Selinexor API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Selinexor as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Selinexor and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Selinexor NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Selinexor suppliers with NDC on PharmaCompass.
Selinexor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Selinexor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Selinexor GMP manufacturer or Selinexor GMP API supplier for your needs.
A Selinexor CoA (Certificate of Analysis) is a formal document that attests to Selinexor's compliance with Selinexor specifications and serves as a tool for batch-level quality control.
Selinexor CoA mostly includes findings from lab analyses of a specific batch. For each Selinexor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Selinexor may be tested according to a variety of international standards, such as European Pharmacopoeia (Selinexor EP), Selinexor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Selinexor USP).

