
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 435, Tmc
2. 435350, Tmc
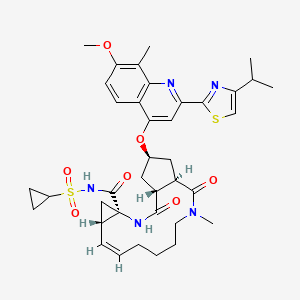
3. N-(17-(2-(4-isopropylthiazole-2-yl)-7-methoxy-8-methylquinolin-4-yloxy)-13-methyl-2,14-dioxo-3,13-diazatricyclo(13.3.0.04,6)octadec-7-ene-4-carbonyl)(cyclopropyl)sulfonamide
4. Olysio
5. Tmc 435
6. Tmc 435350
7. Tmc-435
8. Tmc-435350
9. Tmc435
10. Tmc435350
1. Tmc435
2. 923604-59-5
3. Tmc435350
4. Tmc-435
5. Olysio
6. Tmc 435
7. Tmc 435350
8. Tmc-435350
9. Simeprevir Sodium
10. Chembl501849
11. (1r,4r,6s,7z,15r,17r)-n-cyclopropylsulfonyl-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carboxamide
12. (2r,3ar,10z,11as,12ar,14ar)-n-(cyclopropylsulfonyl)-2-({7-methoxy-8-methyl-2-[4-(1-methylethyl)-1,3-thiazol-2-yl]quinolin-4-yl}oxy)-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1h)-carboxamide
13. Simeprevir [usan]
14. 9ws5rd66hz
15. Simeprevir Sodium [jan]
16. Hcv-pi
17. Simeprevir [mi]
18. Simeprevir [inn]
19. Simeprevir [jan]
20. Simeprevir [vandf]
21. Simeprevir [who-dd]
22. Schembl826061
23. Gtpl7367
24. Dtxsid20919221
25. Chebi:134743
26. N-cyclopropylsulfonyl-[[2-(4-isopropylthiazol-2-yl)-7-methoxy-8-methyl-4-quinolyl]oxy]-methyl-dioxo-[?]carboxamide
27. Bdbm50336504
28. S5015
29. Zinc85540268
30. Akos025401966
31. Ccg-270435
32. Ac-27651
33. As-56205
34. Tmc-00435350
35. Tmc-435, Tmc-435350
36. Us8741926, 47
37. Us8754106, 47
38. Q7517689
39. (2r,3ar,10z,11as,12ar,14ar)-n-(cyclopropylsulfonyl)-2-({7-methoxy-8-methyl-2-[4-(propan-2-yl)-1,3-thiazol-2-yl]quinolin-4-yl}oxy)-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1h)-carboxamide
40. (2r,3ar,10z,12s,13r,15ar)-n-(cyclopropylsulfonyl)-2-[[2-(4-isopropylthiazole-2-yl)-7-methoxy-8-methyl-4-quinolyl]oxy]-5-methyl-4,15-dioxo-2,3,3a,4,5,6,7,8,9,12,13,14,15,15a-tetradecahydro-12,13-methano-5,14-diaza-1h-cyclopentacyclotetradecene-13-carboxamide
41. (2r,3ar,11as,12ar,14ar)-n-(cyclopropylsulfonyl)-2-(2-(4-isopropylthiazol-2-yl)-7-methoxy-8-methylquinolin-4-yloxy)-5-methyl-4,14-dioxo-1,2,3,3a,4,5,6,7,8,9,11a,12,12a,13,14,14a-hexadecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a-carboxamide
42. (2r,3ar,11as,12ar,14ar,z)-n-(cyclopropylsulfonyl)-2-((2-(4-isopropylthiazol-2-yl)-7-methoxy-8-methylquinolin-4-yl)oxy)-5-methyl-4,14-dioxo-1,2,3,3a,4,5,6,7,8,9,11a,12,12a,13,14,14a-hexadecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a-carboxamide
43. (2r,3ar,11as,12ar,14ar,z)-n-(cyclopropylsulfonyl)-2-((2-(4-isopropylthiazol-2-yl)-7-methoxy-8-methylquinolin-4-yl)oxy)-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1h)-carboxamide
44. (2r,3ar,11as,12ar,14ar,z)-n-(cyclopropylsulfonyl)-2-(2-(4-isopropylthiazol-2-yl)-7-methoxy-8-methylquinolin-4-yloxy)-5-methyl-4,14-dioxo-1,2,3,3a,4,5,6,7,8,9,11a,12,12a,13,14,14a-hexadecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a-carboxamide
45. 1217263-04-1
46. N-[17-[2-(4-isopropylthiazole-2-yl)-7-methoxy-8-methylquinolin-4-yloxy]-13-methyl-2,14-dioxo-3,13-diazatricyclo [13.3.0.0''4,6]octadec-7-ene-4-carbonyl](cyclopropyl)sulfonamide
| Molecular Weight | 749.9 g/mol |
|---|---|
| Molecular Formula | C38H47N5O7S2 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 8 |
| Exact Mass | 749.29169120 g/mol |
| Monoisotopic Mass | 749.29169120 g/mol |
| Topological Polar Surface Area | 194 Ų |
| Heavy Atom Count | 52 |
| Formal Charge | 0 |
| Complexity | 1490 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents; Protease Inhibitors
National Library of Medicine's Medical Subject Headings. Simeprevir. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Olysio is a hepatitis C virus (HCV) NS3/4A protease inhibitor indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection as a component of a combination antiviral treatment regimen. /Included in US product label/
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Olysio monotherapy is not recommended.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Olysio combination with peginterferon alfa and ribavirin: screening patients with hepatitis C virus (HCV) genotype 1a infection for the presence of virus with the NS3 Q80K polymorphism is strongly recommended and alternative therapy should be considered if HCV genotype 1a with Q80K is detected.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Olysio is not recommended in patients who have previously failed therapy with a treatment regimen that included Olysio or other hepatitis C virus (HCV) protease inhibitors.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Simeprevir contains a sulfonamide moiety. In clinical trials of simeprevir, an increased incidence of rash or photosensitivity was not observed in the 16 patients who had a history of sulfa allergy. However, data are insufficient to exclude an association between sulfa allergy and the frequency or severity of adverse reactions reported with simeprevir.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 807
During the 12 weeks of treatment with Olysio, dyspnea was reported in 12% of Olysio-treated subjects compared to 8% of placebo-treated subjects (all grades; pooled Phase 3 trials). All dyspnea events reported in Olysio-treated subjects were of mild or moderate severity (Grade 1 or 2). There were no Grade 3 or 4 dyspnea events reported and no subjects discontinued treatment with Olysio due to dyspnea. Sixty-one percent (61%) of dyspnea events occurred in the first 4 weeks of treatment with Olysio.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Adverse effects reported in more than 20% of patients receiving simeprevir in conjunction with peginterferon alfa and ribavirin in clinical trials and occurring with an incidence at least 3% higher than that reported in patients receiving placebo in conjunction with peginterferon alfa and ribavirin include rash (including photosensitivity), pruritus, and nausea.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 808
Rash has been reported in patients receiving simeprevir in conjunction with peginterferon alfa and ribavirin. Rash occurred most frequently during the first 4 weeks of treatment, but can occur at any time during the course of treatment. Rash generally was mild or moderate in severity, but severe rash and rash requiring discontinuance of the drug have been reported. Patients with mild to moderate rash should be monitored for possible progression (e.g., development of oral lesions, conjunctivitis, systemic symptoms). If rash becomes severe, simeprevir should be discontinued. Patients should be monitored until rash resolves.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 807
For more Drug Warnings (Complete) data for Simeprevir (12 total), please visit the HSDB record page.
Olysio is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adult patients.
For hepatitis C virus (HCV) genotype specific activity.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
J05AE14
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AP - Antivirals for treatment of hcv infections
J05AP05 - Simeprevir
Simeprevir is extensively bound to plasma proteins (greater than 99.9%), primarily to albumin and, to a lesser extent, alfa 1-acid glycoprotein. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Administration of simeprevir with food to healthy subjects increased the relative bioavailability (AUC) by 61% and 69% after a high-fat, high-caloric (928 kcal) and normal-caloric (533 kcal) breakfast, respectively, and delayed the absorption by 1 hour and 1.5 hours, respectively. Due to increased bioavailability, Olysio should be administered with food. The type of food does not affect exposure to simeprevir.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Elimination of simeprevir occurs via biliary excretion. Renal clearance plays an insignificant role in its elimination. Following a single oral administration of 200 mg (14)C-simeprevir to healthy subjects, on average 91% of the total radioactivity was recovered in feces. Less than 1% of the administered dose was recovered in urine. Unchanged simeprevir in feces accounted for on average 31% of the administered dose.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
In animals, simeprevir is extensively distributed to gut and liver (liver:blood ratio of 29:1 in rat) tissues. In vitro data and physiologically-based pharmacokinetic modeling and simulations indicate that hepatic uptake in humans is mediated by OATP1B1/3.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
For more Absorption, Distribution and Excretion (Complete) data for Simeprevir (10 total), please visit the HSDB record page.
Following a single oral administration of 200 mg (1.3 times the recommended dosage) (14)C-simeprevir to healthy subjects, the majority of the radioactivity in plasma (mean: 83%) was accounted for by unchanged drug and a small part of the radioactivity in plasma was related to metabolites (none being major metabolites). Metabolites identified in feces were formed via oxidation at the macrocyclic moiety or aromatic moiety or both and by O-demethylation followed by oxidation.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Simeprevir is metabolized in the liver. In vitro experiments with human liver microsomes indicated that simeprevir primarily undergoes oxidative metabolism by the hepatic CYP3A system. Involvement of CYP2C8 and CYP2C19 cannot be excluded. Co-administration of Olysio with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir, and co-administration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
The in vitro metabolism of 14C-TMC435 was investigated in hepatocytes and liver microsomes of mouse, rat, rabbit, monkey and human. The metabolic activity reported in vitro from animals and man was low. Phase II conjugation pathways of Phase I metabolites were formed in hepatocytes. Parent TMC435 was found in much greater levels than any metabolite in vitro. More than 20 metabolites were identified. The metabolic Phase I route of highest importance were O-demethylation of unchanged drug (particularly in animals), oxidation of unchanged drug and oxidized metabolites (particularly in monkey and man) and glucuronidation was the major Phase II of oxidized metabolites (less in human). Only one human metabolite identified in vitro not seen in rat or dog was M22 (oxidized unchanged drug) but this metabolite was identified in rat (feces). In vivo data reveals that the main moiety present in plasma of rat, dog and man was parent TMC435. The major metabolites reported in vivo in plasma from animals and human were M18 and M21. O-desmethyl-TMC435 M21 was the only common circulating metabolite found in rat dog and human plasma (M21: 8% of the mean TMC435 plasma and only small traces in dogs), while M18 was common to plasma of rats and dogs but with respect to the parent compound they appeared with low concentrations (M18: between 28.9% and 12.5% in rats, with only small traces in dogs). Only traces of metabolites M18, M21 and M8 formed by O-demethylation and oxidation at the aromatic moiety were reported in dog plasma. M21 represents less than 10% of unchanged drug and also total radioactivity therefore systemic exposure to M21 was not assessed in the safety evaluation studies. M21 did not appear to accumulate in man. In bile from rats, moderately high levels of parent compound were reported (0.11 to 17.2%). TMC435 metabolites in this matrix were formed mainly by hydroxylation and O-demethylation and also by glucuronidation.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Olysio (Simeprevir); p.19-20 (March 20, 2014). Available from, as of March 23, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002777/WC500167870.pdf
The most important metabolic route TMC435 in rat and dog was O-demethylation of the parent drug to M18 (12.8%- 6.4% male-female rats; 18.8% dogs). In rats other metabolites were formed by oxidation of M18 and oxidation of unchanged drug. In dogs, further oxidation of M18 to M14 and M8, and of the unchanged drug to M21, M16 and M11 were also reported as minor routes. The human metabolism profile suggests that TMC435 is mainly metabolized by two main routes, (1) oxidation of unchanged drug, either at the macrocyclic moiety (M27, M21 and M22), or at the aromatic moiety (M26 and M16), or both (M23, M24, M25 and M11) and (2) the O-demethylation of unchanged drug to M18, followed by oxidation on the macrocyclic moiety to M14 and by oxidation on the aromatic moiety to M5, appears to be the secondary metabolic pathway in man. M21 and M22 were the most important metabolites in human faeces. Other relevant metabolites (1% of the dose) were M11, M16, M27 and M18. All metabolites detected in human feces were detected in vitro and/or in vivo in rat and/or dog feces. The main CYP enzymes involved in TMC435 metabolism were CYP3A enzymes although in vitro data suggests the involvement of CYP2C8 and CYP2C19.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Olysio (Simeprevir); p.20 (March 20, 2014). Available from, as of March 23, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002777/WC500167870.pdf
The half-life was variable among species accounting to 4.0 hr in rats, 3.7 hr in rabbits and dogs and 5 to 6 hr in Rhesus and Cynomolgus monkeys.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Olysio (Simeprevir); p.19 (March 20, 2014). Available from, as of March 23, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002777/WC500167870.pdf
The terminal elimination half-life of simeprevir was 10 to 13 hours in hepatitis C virus (HCV)-uninfected subjects and 41 hours in HCV-infected subjects receiving 200 mg (1.3 times the recommended dosage) of simeprevir.
NIH; DailyMed. Current Medication Information for Olysio (Simeprevir) Capsule (Revised: November 2014). Available from, as of March 18, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1816fd68-0ed7-4a37-84bb-e298c5ab6e28
Simeprevir sodium is a selective hepatitis C virus (HCV) nonstructural 3/4A (NS3/4A) protease inhibitor (PI). The drug is a direct-acting antiviral (DAA) with activity against HCV. Simeprevir binds noncovalently to the active site of HCV NS3/4A protease, thereby blocking enzyme activity essential for viral replication (i.e., cleavage of the HCV-encoded polyprotein at the NS3/NS4A, NS4A/NS4B, NS4A/NS5A, and NS5A/NS5B junctions). In vitro studies using biochemical and cell-based replicon assays indicate that simeprevir has potent activity against HCV genotypes 1a and 1b1 5 8 9 and has some activity against HCV genotypes 2, 4, 5, and 6 (not genotype 3).
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 809
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
51
PharmaCompass offers a list of Simeprevir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Simeprevir manufacturer or Simeprevir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Simeprevir manufacturer or Simeprevir supplier.
PharmaCompass also assists you with knowing the Simeprevir API Price utilized in the formulation of products. Simeprevir API Price is not always fixed or binding as the Simeprevir Price is obtained through a variety of data sources. The Simeprevir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Simeprevir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Simeprevir, including repackagers and relabelers. The FDA regulates Simeprevir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Simeprevir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Simeprevir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Simeprevir supplier is an individual or a company that provides Simeprevir active pharmaceutical ingredient (API) or Simeprevir finished formulations upon request. The Simeprevir suppliers may include Simeprevir API manufacturers, exporters, distributors and traders.
click here to find a list of Simeprevir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Simeprevir DMF (Drug Master File) is a document detailing the whole manufacturing process of Simeprevir active pharmaceutical ingredient (API) in detail. Different forms of Simeprevir DMFs exist exist since differing nations have different regulations, such as Simeprevir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Simeprevir DMF submitted to regulatory agencies in the US is known as a USDMF. Simeprevir USDMF includes data on Simeprevir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Simeprevir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Simeprevir suppliers with USDMF on PharmaCompass.
Simeprevir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Simeprevir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Simeprevir GMP manufacturer or Simeprevir GMP API supplier for your needs.
A Simeprevir CoA (Certificate of Analysis) is a formal document that attests to Simeprevir's compliance with Simeprevir specifications and serves as a tool for batch-level quality control.
Simeprevir CoA mostly includes findings from lab analyses of a specific batch. For each Simeprevir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Simeprevir may be tested according to a variety of international standards, such as European Pharmacopoeia (Simeprevir EP), Simeprevir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Simeprevir USP).
