
Synopsis
Synopsis
0
NDC API
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
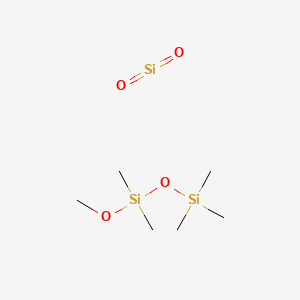

1. Phazyme 125
1. 8050-81-5
2. Dioxosilane;methoxy-dimethyl-trimethylsilyloxysilane
3. Simeticone
4. Schembl339371
5. Db09512
6. Ft-0674588
7. Q419415
1. Simethicone Usp
| Molecular Weight | 238.46 g/mol |
|---|---|
| Molecular Formula | C6H18O4Si3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 238.05128865 g/mol |
| Monoisotopic Mass | 238.05128865 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 125 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antifoaming Agents; Emollients
National Library of Medicine's Medical Subject Headings. Simethicone. Online file (MeSH, 2015). Available from, as of October 16, 2015: https://www.nlm.nih.gov/cgi/mesh/2015/MB_cgi
/EXPERIMENTAL THERAPY/ Currently, there is no standardized protocol for bowel preparation before small bowel capsule endoscopy (SBCE). This study aimed to investigate the effect of simethicone combined with polyethylene glycol (PEG) on the visualization quality (VQ) of the SBCE in patients with or without known or suspected Crohn's disease (CD). This observational, prospective, single-center study included consecutive patients undergoing a SBCE between 2007 and 2008. Patients received either a standard bowel cleansing preparation of 2 L PEG and 80 mg simethicone orally 12 and 1 h before SBCE respectively (Group A) or only PEG (Group B). VQ, based on scores for luminal bubbles in frames taken from the small intestine, examination completeness, SBCE diagnostic yield, gastric and small bowel transit times were recorded. Of the 115 patients finally included (Group A, n=56 and Group B, n=59) the cecum was visualized in 103 (89.6%). Simethicone overall improved the VQ in the proximal [OR: 2.43 (95%CI: 1.08-5.45), P=0.032] but not in the distal bowel segment (P=0.064). Nevertheless, this effect was not observed in patients undergoing SBCE for either known or suspected CD. Simethicone as an adjunct to PEG for bowel preparation in patients undergoing SBCE significantly improved the VQ in non-CD patients.
PMID:26423317 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4585393 Papamichael K et al; Ann Gastroenterol 28 (4): 464-8 (2015)
Simethicone is used as an adjunct in the symptomatic treatment of flatulence, functional gastric bloating, and postoperative gas pains. For self-medication, the drug is used as an antiflatulent to relieve symptoms commonly referred to as gas, including upper GI bloating, pressure, fullness, or stuffed feeling. Simethicone also has been used prior to gastroscopy to enhance visualization and prior to radiography of the intestine to reduce gas shadows. Although there is gastroscopic evidence that simethicone aids in the elimination of gas from the GI tract and reduces postoperative gas pains, the relationship of gas accumulation to what patients commonly refer to as symptoms of gas under ordinary conditions is not clear; however, the drug also has been shown to be effective in relieving these symptoms. Preparations of simethicone with antacids, antispasmodics, or digestive enzymes are available, but use of inflexible combinations of drugs is often unwarranted, and these products have not been well evaluated.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Although simethicone is an effective antiflatulent, there currently is no conclusive evidence that immediate postprandial upper abdominal distress (IPPUAD) is caused by excessive gas, despite the fact that many patients commonly attribute symptoms of the distress to gas. In addition, current data are insufficient to establish the efficacy of simethicone for the symptomatic relief of IPPUAD, a symptom complex that occurs within 30 minutes after a meal and consists of sensations of GI bloating, distention, fullness, or pressure with upper abdominal discomfort but not aerophagia or hyperacidity.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
For more Therapeutic Uses (Complete) data for Simethicone (11 total), please visit the HSDB record page.
... Simethicone and carbamazepine, when taken together, may be a cause of carbamazepine toxicity. The risk of carbamazepine overdose should be considered when prescribing simethicone to a patient who is using carbamazepine.
PMID:18652684 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2495000 Guneysel O et al; J Med Case Reports 2: 242 (2008)
Although no data are available on the use of simethicone during breastfeeding, it is known that simethicone is not absorbed orally. Therefore, it cannot be transferred to breastmilk. It is also used safely in breastfed infants. No special precautions are required.
LacMed; Antifoaming Agents Record on Simethicone (8050-81-5) (LactMed Record 536) revised on September 7, 2013. Available from, as of October 22, 2015: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+536
Simethicone is apparently nontoxic, and no adverse effects have been reported.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Simethicone is indicated for the treatment of bloating, pressure, and cramps caused by gas. Simethicone is also used as part of bowel preparation for colonoscopies.
Simethicone decreases the surface tension of gas bubbles in the gastrointestinal tract, facilitating their expulsion. It has a short duration of action as it is generally given as needed, and a wide therapeutic index as it is not systemically absorbed.
Emollients
Oleagenous substances used topically to soothe, soften or protect skin or mucous membranes. They are used also as vehicles for other dermatologic agents. (See all compounds classified as Emollients.)
Antifoaming Agents
Agents used to prevent the formation of foam or to treat flatulence or bloat. (See all compounds classified as Antifoaming Agents.)
Absorption
Simethicone is not systemically absorbed and so these data are not readily available.
Route of Elimination
Simethicone is eliminated in the feces.
Volume of Distribution
Simethicone is not systemically absorbed and so these data are not readily available.
Clearance
Simethicone is not systemically absorbed and so these data are not readily available.
Simethicone is physiologically inert; it does not appear to be absorbed from the GI tract or to interfere with gastric secretion or absorption of nutrients. Following oral administration, the drug is excreted unchanged in feces.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Simethicone is not systemically absorbed and so it is not metabolised by the body.
Simethicone is not systemically absorbed and so these data are not readily available.
Simethicone is a surfactant that decreases the surface tension of gas bubbles in the gastrointestinal tract, more easily allowing gas to exit the body.
The clinical use of simethicone is based on its antifoam properties. Silicone antifoams spread on the surface of aqueous liquids, forming a film of low surface tension and thus causing collapse of foam bubbles. Simethicone reportedly allows mucus-surrounded gas bubbles in the GI tract to coalesce and be expelled.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
96
PharmaCompass offers a list of Simethicone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Simethicone manufacturer or Simethicone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Simethicone manufacturer or Simethicone supplier.
PharmaCompass also assists you with knowing the Simethicone API Price utilized in the formulation of products. Simethicone API Price is not always fixed or binding as the Simethicone Price is obtained through a variety of data sources. The Simethicone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Simethicone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Simethicone, including repackagers and relabelers. The FDA regulates Simethicone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Simethicone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Simethicone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Simethicone supplier is an individual or a company that provides Simethicone active pharmaceutical ingredient (API) or Simethicone finished formulations upon request. The Simethicone suppliers may include Simethicone API manufacturers, exporters, distributors and traders.
click here to find a list of Simethicone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Simethicone DMF (Drug Master File) is a document detailing the whole manufacturing process of Simethicone active pharmaceutical ingredient (API) in detail. Different forms of Simethicone DMFs exist exist since differing nations have different regulations, such as Simethicone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Simethicone DMF submitted to regulatory agencies in the US is known as a USDMF. Simethicone USDMF includes data on Simethicone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Simethicone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Simethicone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Simethicone Drug Master File in Japan (Simethicone JDMF) empowers Simethicone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Simethicone JDMF during the approval evaluation for pharmaceutical products. At the time of Simethicone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Simethicone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Simethicone Drug Master File in Korea (Simethicone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Simethicone. The MFDS reviews the Simethicone KDMF as part of the drug registration process and uses the information provided in the Simethicone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Simethicone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Simethicone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Simethicone suppliers with KDMF on PharmaCompass.
A Simethicone CEP of the European Pharmacopoeia monograph is often referred to as a Simethicone Certificate of Suitability (COS). The purpose of a Simethicone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Simethicone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Simethicone to their clients by showing that a Simethicone CEP has been issued for it. The manufacturer submits a Simethicone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Simethicone CEP holder for the record. Additionally, the data presented in the Simethicone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Simethicone DMF.
A Simethicone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Simethicone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Simethicone suppliers with CEP (COS) on PharmaCompass.
A Simethicone written confirmation (Simethicone WC) is an official document issued by a regulatory agency to a Simethicone manufacturer, verifying that the manufacturing facility of a Simethicone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Simethicone APIs or Simethicone finished pharmaceutical products to another nation, regulatory agencies frequently require a Simethicone WC (written confirmation) as part of the regulatory process.
click here to find a list of Simethicone suppliers with Written Confirmation (WC) on PharmaCompass.
Simethicone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Simethicone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Simethicone GMP manufacturer or Simethicone GMP API supplier for your needs.
A Simethicone CoA (Certificate of Analysis) is a formal document that attests to Simethicone's compliance with Simethicone specifications and serves as a tool for batch-level quality control.
Simethicone CoA mostly includes findings from lab analyses of a specific batch. For each Simethicone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Simethicone may be tested according to a variety of international standards, such as European Pharmacopoeia (Simethicone EP), Simethicone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Simethicone USP).

