
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
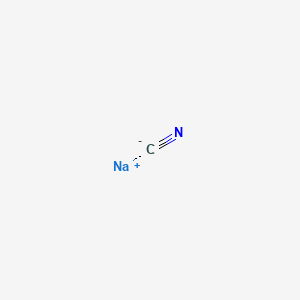

1. Cyanide, Sodium
2. Cyanogran
1. 143-33-9
2. Cymag
3. Sodium Cyanide (na(cn))
4. Cyanide Of Sodium
5. Nacn
6. Cyanasalt H
7. Cyanasalt S
8. Sodium;cyanide
9. Cyanide Salts
10. Kyanid Sodny
11. Sodium Cyanide [iso]
12. Hydrocyanic Acid, Sodium Salt
13. Cianuro Di Sodio
14. Cyanure De Sodium
15. Cyanosodium
16. Sodium Cyanide Solution
17. Cyanogran
18. O5ddb9z95g
19. Chembl1644697
20. Nsc-77379
21. Cyanobrik
22. Caswell No. 758
23. Kyanid Sodny [czech]
24. M-44 Cyanide Capsules
25. Rcra Waste Number P106
26. Cianuro Di Sodio [italian]
27. Cyanure De Sodium [french]
28. Ccris 7712
29. Hsdb 734
30. Cyanure De Sodium [iso-french]
31. Einecs 205-599-4
32. Nsc 77379
33. Un1689
34. Rcra Waste No. P106
35. Unii-o5ddb9z95g
36. Epa Pesticide Chemical Code 074002
37. Brn 3587243
38. Natriumcyanid
39. Natriumzyanid
40. Sodium-cyanide
41. Cyano Sodium
42. Sodium Cyanide-
43. Sodium Cyanide, Solid
44. Sodium Cyanide, Granular
45. Sodium Cyanide Acs Grade
46. Sodium Cyanide (solution)
47. Wln: Na Cn
48. Ec 205-599-4
49. Wln: Nc-na-
50. Sodium Cyanide [mi]
51. Sodium Cyanide [hsdb]
52. Dtxsid4024309
53. Chebi:33192
54. Nsc77379
55. Sodium Cyanide, Reagent Grade, 97%
56. Akos009159016
57. Sodium Cyanide [un1689] [poison]
58. Sodium Cyanide, Acs Reagent, >=95.0%
59. Sodium Cyanide, Saj First Grade, >=90.0%
60. C18673
61. Sodium Cyanide, Jis Special Grade, >=97.0%
62. Sodium Cyanide, Purum P.a., >=96.0% (at)
63. Q410185
64. Sodium Cyanide, Puriss. P.a., Acs Reagent, >=97.0% (at)
| Molecular Weight | 49.007 g/mol |
|---|---|
| Molecular Formula | CNNa |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 48.99284329 g/mol |
| Monoisotopic Mass | 48.99284329 g/mol |
| Topological Polar Surface Area | 23.8 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 12.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
Poisons
Substances which, when ingested, inhaled, or absorbed, or when applied to, injected into, or developed within the body in relatively small amounts may, by their chemical action, cause damage to structure or disturbance of function. (From Dorland, 27th ed) (See all compounds classified as Poisons.)
IN 30 DAYS, 72% OF (14)C FROM AN IP DOSE OF (14)C-CYANIDE TO MICE WAS EXCRETED IN URINE AND FECES, 25% IN EXPIRED AIR AND 3% WAS RETAINED IN ANIMALS. PEAK EXCRETION OCCURRED WITHIN 10 MIN IN EXPIRED AIR AND WITHIN 6-24 HR IN URINE AND FECES. /CYANIDE/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 94
CYANIDES ARE RAPIDLY ABSORBED FROM SKIN & ALL MUCOSAL SURFACES & ARE MOST DANGEROUS WHEN INHALED, BECAUSE TOXIC AMT ARE ABSORBED THROUGH BRONCHIAL MUCOSA & ALVEOLI. /CYANIDES/
Haddad, L.M. and Winchester, J.F. Clinical Management of Poisoning and Drug Overdosage. Philadelphia, PA: W.B. Saunders Co., 1983., p. 745
THE CYANIDE ION IS READILY ABSORBED AFTER ORAL OR PARENTERAL ADMIN. PROLONGED LOCAL CONTACT WITH CYANIDE SOLN ... MAY RESULT IN ABSORPTION OF TOXIC AMT THROUGH SKIN. PART OF ABSORBED CYANIDE IS EXCRETED UNCHANGED BY THE LUNG. LARGER PORTION ... IS CONVERTED BY SULFURTRANSFERASE RELATIVELY NONTOXIC TO THIOCYANATE ION. /CYANIDE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 904
As estimated in rats given 30 mg sodium cyanide intraperitoneally over a period of 8 days, 80 percent of the total cyanide is excreted in the urine in the form of thiocyanate.
Wood JL, Cooley SL; J Biol Chem 218: 449 (1956) as cited in USEPA; Ambient Water Quality Criteria Doc: Cyanides p.C-14 (1980) EPA 440/5-80-037
For more Absorption, Distribution and Excretion (Complete) data for SODIUM CYANIDE (9 total), please visit the HSDB record page.
OPOSSUMS WERE DOSED WITH SODIUM CYANIDE BY ... STOMACH TUBE. ... ANALYSIS INDICATED THAT MAJOR ROUTE OF DETOXICATION ... WAS BY CONVERSION TO THIOCYANATE, WHICH WAS EXCRETED IN URINE. TRACES OF 2-IMINO-4-THIAZOLIDINE CARBOXYLIC ACID WERE OBSERVED IN CRUDE CONCENTRATED EXTRACT OF URINE.
Menzie, C.M. Metabolism of Pesticides, Update II. U.S. Department of the Interior, Fish Wildlife Service, Special Scientific Report - Wildlife No. 2l2. Washington, DC: U.S. Government Printing Office, 1978., p. 85
... CYANIDE ION IS CONJUGATED WITH SULFUR TO FORM THIOCYANATE. ... CONJUGATION IS CATALYZED BY THE ENZYME RHODANESE WHICH IS WIDELY DISTRIBUTED IN MOST ANIMAL TISSUES EXCEPT BLOOD, LIVER BEING PARTICULARLY ACTIVE. ... THE RHODANESE MECHANISM IS CAPABLE OF DETOXICATING ONLY LIMITED AMT OF CYANIDE, SUCH AS ARE FORMED DURING NORMAL METAB. /ANOTHER SULFUR DONOR IS 3-MERCAPTOPYRUVATE. THE ENZYME, MERCAPTOSULFUR TRANSFERASE IS LOCALIZED IN CYTOSOL./ /CYANIDE/
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 96
Salmonella typhimurium strain (OASS positive) (OASS, O-acetylserine sulfhydrylase) synthesize a toxic but non-mutagenic metabolite from cyanide and O-acetylserine. Salmonella typhimurium mutant DW379 (OASS deficient) is neither able to carry out this reaction in vitro nor produce the toxic metabolite in vivo. L-Cysteine reverses the cyanide metabolite mediated inhibition and thus allows OASS positive strains to grow in medium containing cyanide and O-acetylserine. The results suggest that the enzyme O-acetylserine sulfhydrylase catalyzes the reaction of cyanide and O-acetylserine to form the toxic metabolite. This metabolite from ninhydrin-positive, adheres strongly to the cation-exchange column, and migrates in thin layer chromatography to an Rf value similar to that of beta-cyanoalanine.
PMID:3932844 Owais WM et al; Mutat Res 144 (3): 119-26 (1985)
/ONE OF/ THE MAJOR MECHANISMS FOR REMOVING CYANIDE FROM THE BODY IS ITS ENZYMATIC CONVERSION, BY THE MITOCHONDRIAL ENZYME RHODANESE (TRANSSULFURASE), TO THIOCYANATE, WHICH IS RELATIVELY ... /LESS TOXIC/. /CYANIDE/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1643
For more Metabolism/Metabolites (Complete) data for SODIUM CYANIDE (6 total), please visit the HSDB record page.
Half-life for the conversion of cyanide to thiocyanate from a non-lethal dose in man is between 20 min and 1 hr. /Cyanide/
Feldstein M, Klendshoj NC; J Lab Chin Med 44: 166-70 (1954) as cited in NIOSH; Criteria Document: Hydrogen Cyanide and Cyanide Salts p.45 (1976) DHEW Pub. NIOSH 77-108
CYANIDE HAS A VERY HIGH AFFINITY FOR IRON IN THE FERRIC STATE. WHEN ABSORBED, /CYANIDE/ ... REACTS READILY WITH TRIVALENT IRON OF CYTOCHROME OXIDASE IN MITTCHONDRIA; CELLULAR RESPIRATION IS THUS INHIBITED & CYTOTOXIC HYPOXIA RESULTS. SINCE UTILIZATION OF OXYGEN IS BLOCKED, VENOUS BLOOD IS OXYGENATED AND IS ALMOST AS BRIGHT RED AS ARTERIAL BLOOD. RESPIRATION IS STIMULATED BECAUSE CHEMORECEPTIVE CELLS RESPOND AS THEY DO TO DECREASED OXYGEN. A TRANSIENT STAGE OF CNS STIMULATION WITH HYPERPNEA AND HEADACHE IS OBSERVED; FINALLY THERE ARE HYPOXIC CONVULSIONS AND DEATH DUE TO RESPIRATORY ARREST. /CYANIDE/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 1642
SINGLE DOSES OF CYANIDE PRODUCE ALTERATIONS IN PATTERN OF BRAIN METABOLITES CONSISTENT WITH DECR IN OXIDATIVE METABOLISM & INCR IN GLYCOLYSIS. DECR IN BRAIN GAMMA-AMINOBUTYRIC ACID ... HAVE BEEN ASCRIBED TO CYANIDE INHIBITION OF GLUTAMIC ACID DECARBOXYLASE. /CYANIDE/
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. III-126
THE CORTICAL GRAY MATTER, HIPPOCAMPUS (H1), CORPORA STRIATA, & SUBSTANTIA NIGRA ARE COMMONLY AFFECTED. ... CYANIDE ALSO HAS PROPENSITY FOR DAMAGING WHITE MATTER, PARTICULARLY CORPUS CALLOSUM. CYANIDE INHIBITS CYTOCHROME OXIDASE & PRODUCES CYTOTOXIC ANOXIA, BUT ALSO CAUSES HYPOTENSION THROUGH ITS EFFECTS ON HEART. /CYANIDE/
Doull, J., C.D.Klassen, and M.D. Amdur (eds.). Casarett and Doull's Toxicology. 3rd ed., New York: Macmillan Co., Inc., 1986., p. 372
Evoked release of transmitter at the squid giant synapse was examined under conditions where the calcium ion concentration in the presynaptic terminal was manipulated by inhibitors of calcium sequestration. Simultaneous intracellular recordings of presynaptic and postsynaptic resting action potentials were made during bath application of various metabolic inhibitors including sodium cyanide. Cyanide reversibly depressed the post-synaptic potential. The progressive reduction of post-synaptic potential amplitude was accompanied by a reversible increase in synaptic delay. The time course of block of the post-synaptic potential was similar for different agents and dependant on the rate of presynaptic activity (30-40 min at 0.01 Hz). Recovery of the post-synaptic action potential following block by cyanide was obtained within 40 min. Synaptic depression by the metabolic inhibitors does not result from changes in the presynaptic resting or action potentials, nor from a change in post-synaptic receptor sensitivity. The post-synaptic response to local ionophoresis of L-glutamate was unchanged following the inhibition of evoked release of transmitter by cyanide. Injections of EDTA into presynaptic terminals poisoned by cyanide produced transient increases in post-synaptic potential amplitude, suggesting that cyanide is having its effect through raising intracellular calcium rather than lowering ATP. Control experiments injecting EDTA into unpoisoned nerve terminals showed no apparent effect on evoked transmitter release.
PMID:2419546 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1192641 Adams DJ et al; J Physiol 369: 145-159 (1985)
For more Mechanism of Action (Complete) data for SODIUM CYANIDE (10 total), please visit the HSDB record page.
Market Place

ABOUT THIS PAGE
68
PharmaCompass offers a list of Sodium Cyanide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Cyanide manufacturer or Sodium Cyanide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Cyanide manufacturer or Sodium Cyanide supplier.
PharmaCompass also assists you with knowing the Sodium Cyanide API Price utilized in the formulation of products. Sodium Cyanide API Price is not always fixed or binding as the Sodium Cyanide Price is obtained through a variety of data sources. The Sodium Cyanide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sodium Cyanide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sodium Cyanide, including repackagers and relabelers. The FDA regulates Sodium Cyanide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sodium Cyanide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Sodium Cyanide supplier is an individual or a company that provides Sodium Cyanide active pharmaceutical ingredient (API) or Sodium Cyanide finished formulations upon request. The Sodium Cyanide suppliers may include Sodium Cyanide API manufacturers, exporters, distributors and traders.
Sodium Cyanide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sodium Cyanide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sodium Cyanide GMP manufacturer or Sodium Cyanide GMP API supplier for your needs.
A Sodium Cyanide CoA (Certificate of Analysis) is a formal document that attests to Sodium Cyanide's compliance with Sodium Cyanide specifications and serves as a tool for batch-level quality control.
Sodium Cyanide CoA mostly includes findings from lab analyses of a specific batch. For each Sodium Cyanide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sodium Cyanide may be tested according to a variety of international standards, such as European Pharmacopoeia (Sodium Cyanide EP), Sodium Cyanide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sodium Cyanide USP).
