



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
Listed Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
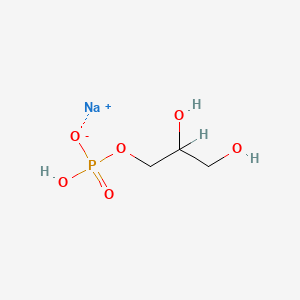

1. 17603-42-8
2. 1,2,3-propanetriol, 1-(dihydrogen Phosphate), Sodium Salt
3. Sodium;2,3-dihydroxypropyl Hydrogen Phosphate
4. Sodium Alpha Glycerophosphate
5. Einecs 241-577-0
6. Alpha-natrium Glycerophosphat [german]
7. Alpha-natrium Glycerophosphat
8. 2,3-dihydroxypropyl (dihydrogen Phosphate), Sodium Salt
9. Disodium,1,3-dihydroxypropan-2-yl Phosphate,hydrate
10. Glycerol, 1-(dihydrogen Phosphate), Sodium Salt
11. Sodiumglycerophosphate
12. Sodium Alpha-glycerophosphate
13. 1,2,3-propentriol, 1-(dihydrogen Phosphate), Sodium Salt
14. Dtxsid70938740
15. Einecs 254-713-9
16. Akos006346295
17. Ft-0696352
18. A913951
19. 1,2,3-propanetriol, Mono(dihydrogen Phosphate), Sodium Salt
20. 1,2,3-propanetriol, 1-(dihydrogen Phosphate), Sodium Salt (1:?)
| Molecular Weight | 194.06 g/mol |
|---|---|
| Molecular Formula | C3H8NaO6P |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 193.99561925 g/mol |
| Monoisotopic Mass | 193.99561925 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |




