
Synopsis
Synopsis
0
FDA Orange Book

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
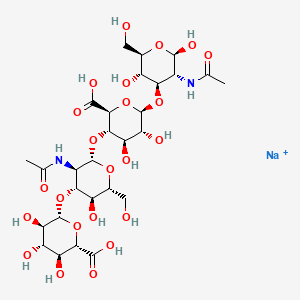

1. Acid, Hyaluronic
2. Amo Vitrax
3. Amvisc
4. Biolon
5. Etamucine
6. Healon
7. Hyaluronate Sodium
8. Hyaluronate, Sodium
9. Hyaluronic Acid
10. Hyvisc
11. Luronit
12. Vitrax, Amo
1. Hyaluronic Acid, Sodium Salt
2. Hyaluronic Acid Sodium
3. Kopuron
4. Healon
5. Hyalgan
6. Hyalurone Sodium
7. 6-[3-acetamido-2-[6-[3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic Acid
8. Sodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic Acid
9. Equron (veterinary)
10. Synacid (veterinary)
11. Arthrease
12. Cystistat
13. Hyalart
14. Hyalein
15. Hyalovet
16. Hyladerm
17. Khionat
18. Monovisc
19. Nidelon
20. Orthovisc
21. Ostenil
22. Provisc
23. Sinovial
24. Supartz
25. Suvenyl
26. Hyasol
27. Hyladerm Khionat
28. Nrd101
29. Ha
30. Hyaluronsan Ha-lq
31. Bio Hyaluro 12
32. Euflexxa Injection
33. Euflexxa
34. Ccris 4127
35. Hyaluronate Sodium [usan:jan]
36. Sodium Hyaluronate Hmw
37. Unii-yse9ppt4th
38. Si-4402
39. Sl-1010
40. Synacid
41. Equron
42. Chlamyhyaluronic Acid Sodium Salt
43. Sl 1010
44. Hyalauronic Acid 99%
45. Yse9ppt4th
46. Gtpl4954
47. Sodium Hyaluronate (food Grade)
48. Sodium Hyaluronate (cosmetic Grade)
49. Akos015896610
50. Sodium Hyaluronate, Low Molecular Weight
51. Sodium Hyaluronate, High Molecular Weight
52. Q27078001
53. (3s,4r,5r,6r)-3-[(2s,3r,5s,6r)-3-acetamido-4-[(2s,3r,4s,6s)-6-carboxy-3,4,5-trihydroxy-tetrahydropyran-2-yl]oxy-5-hydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-6-[(3r,4r,5s,6r)-3-acetamido-2,5-dih
54. Sodium (2s,3s,4r,5r,6r)-3-{[(2s,3r,4r,5s,6r)-4-{[(2r,3r,4s,5s,6s)-6-carboxy-3,4,5-trihydroxyoxan-2-yl]oxy}-3-acetamido-5-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4,5-dihydroxyoxane-2-carboxylic Acid
| Molecular Weight | 799.6 g/mol |
|---|---|
| Molecular Formula | C28H44N2NaO23+ |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 23 |
| Rotatable Bond Count | 12 |
| Exact Mass | 799.22325494 g/mol |
| Monoisotopic Mass | 799.22325494 g/mol |
| Topological Polar Surface Area | 400 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 1 |
| Complexity | 1300 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 20 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
Viscosupplements
Viscoelastic solutions that are injected into JOINTS in order to alleviate symptoms of joint-related disorders such as OSTEOARTHRITIS. (See all compounds classified as Viscosupplements.)
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24507
Submission : 2010-12-20
Status : Active
Type : II

Certificate Number : CEP 2015-200 - Rev 01
Issue Date : 2024-03-05
Type : Chemical
Substance Number : 1472
Status : Valid

Registration Number : 304MF10049
Registrant's Address : No. 678 Tianchen St. , High-Tech Development Zone, Jinan 250101 P. R. China
Initial Date of Registration : 2022-03-02
Latest Date of Registration : --

NDC Package Code : 67828-0000
Start Marketing Date : 2014-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2022-09-02
Registration Number : 20211021-96-E-171-54(2)
Manufacturer Name : Bloomage Biotechnology Corp., Ltd
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan, Shandong province, China
 Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14727
Submission : 2000-02-29
Status : Active
Type : II

Certificate Number : R0-CEP 2018-037 - Rev 01
Issue Date : 2021-03-24
Type : Chemical and TSE
Substance Number : 1472
Status : Valid

Registration Number : 228MF10141
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2016-08-02
Latest Date of Registration : --

Registrant Name : Daeshin Pharmaceutical Co., Ltd.
Registration Date : 2023-06-22
Registration Number : 20071228-96-E-25-08(5)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315
 HTL Biotechnology is the world leader in the responsible development and production of pharmaceutical-grade biopolymers.
HTL Biotechnology is the world leader in the responsible development and production of pharmaceutical-grade biopolymers.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34855
Submission : 2020-10-08
Status : Active
Type : II

Certificate Number : CEP 2004-238 - Rev 06
Issue Date : 2024-07-19
Type : Chemical
Substance Number : 1472
Status : Valid

Registration Number : 228MF10072
Registrant's Address : 7 rue Alfred Kastler-ZI de l'Aumaillerie-35133 JAVENE-France
Initial Date of Registration : 2016-03-07
Latest Date of Registration : --

NDC Package Code : 51035-001
Start Marketing Date : 2014-12-16
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Humedics Co., Ltd.
Registration Date : 2020-06-29
Registration Number : 20080530-96-E-69-18(2)
Manufacturer Name : HTL
Manufacturer Address : 7 Rue Alfred Kastler ZI De L'Aumaillerie 35133 JAVENE
 Topscience Biotech specializes in R&D, production, and sales of Sodium Hyaluronate, focusing on Medical and Pharmaceutical Grade HA.
Topscience Biotech specializes in R&D, production, and sales of Sodium Hyaluronate, focusing on Medical and Pharmaceutical Grade HA.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37918
Submission : 2023-05-05
Status : Active
Type : II

Certificate Number : R1-CEP 2013-349 - Rev 01
Issue Date : 2022-03-23
Type : Chemical
Substance Number : 1472
Status : Valid

Date of Issue : 2022-12-06
Valid Till : 2025-12-18
Written Confirmation Number : SD220042
Address of the Firm :

NDC Package Code : 84109-001
Start Marketing Date : 2024-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Bansen Co., Ltd.
Registration Date : 2021-03-16
Registration Number : 20210316-96-E-168-52
Manufacturer Name : Shandong Topscience Biotech Co., Ltd.
Manufacturer Address : No.98 Lanshan West Road, Lanshan District, Rizhao City, ShandongProvince, PR
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.

Registration Number : 219MF10034
Registrant's Address : 50 Binney Street, Cambridge, MA 02142, USA
Initial Date of Registration : 2007-02-05
Latest Date of Registration : --

Registrant Name : Korea Alcon Co., Ltd.
Registration Date : 2008-05-07
Registration Number : 20080507-96-E-55-14
Manufacturer Name : Genzyme Corporation
Manufacturer Address : 76 New York Avenue, Framingham, MA 01701-9322
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24508
Submission : 2010-12-20
Status : Active
Type : II

Certificate Number : CEP 2010-289 - Rev 04
Issue Date : 2023-09-15
Type : Chemical
Substance Number : 1472
Status : Valid

NDC Package Code : 67828-0000
Start Marketing Date : 2014-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2022-09-02
Registration Number : 20211021-96-E-171-54(2)
Manufacturer Name : Bloomage Biotechnology Corp., Ltd
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan, Shandong province, China
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26039
Submission : 2012-05-07
Status : Active
Type : II

Certificate Number : R1-CEP 2016-172 - Rev 00
Issue Date : 2023-05-02
Type : Chemical
Substance Number : 1472
Status : Valid

NDC Package Code : 67828-0000
Start Marketing Date : 2014-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2022-09-02
Registration Number : 20211021-96-E-171-54(2)
Manufacturer Name : Bloomage Biotechnology Corp., Ltd
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan, Shandong province, China
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26360
Submission : 2012-08-20
Status : Active
Type : II

Certificate Number : CEP 2010-290 - Rev 04
Issue Date : 2024-07-25
Type : Chemical
Substance Number : 1472
Status : Valid

NDC Package Code : 67828-0000
Start Marketing Date : 2014-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2022-09-02
Registration Number : 20211021-96-E-171-54(2)
Manufacturer Name : Bloomage Biotechnology Corp., Ltd
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan, Shandong province, China
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : CEP 2021-220 - Rev 02
Status : Valid
Issue Date : 2024-06-12
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : R0-CEP 2021-446 - Rev 01
Status : Valid
Issue Date : 2023-06-15
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : CEP 2010-289 - Rev 04
Status : Valid
Issue Date : 2023-09-15
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : R1-CEP 2015-201 - Rev 00
Status : Valid
Issue Date : 2021-11-05
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : CEP 2018-107 - Rev 04
Status : Valid
Issue Date : 2024-02-19
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : R0-CEP 2021-258 - Rev 02
Status : Valid
Issue Date : 2023-03-08
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : CEP 2015-200 - Rev 01
Status : Valid
Issue Date : 2024-03-05
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : R1-CEP 2016-172 - Rev 00
Status : Valid
Issue Date : 2023-05-02
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : CEP 2017-195 - Rev 01
Status : Valid
Issue Date : 2024-05-02
Type : Chemical
Substance Number : 1472
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Certificate Number : R0-CEP 2022-129 - Rev 01
Status : Valid
Issue Date : 2023-05-31
Type : Chemical
Substance Number : 1472
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]  Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Purified sodium hyaluronate JP-AE
Registration Number : 304MF10049
Registrant's Address : No. 678 Tianchen St. , High-Tech Development Zone, Jinan 250101 P. R. China
Initial Date of Registration : 2022-03-02
Latest Date of Registration : 2024-02-07
 Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Registration Number : 217MF10439
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2005-08-19
Latest Date of Registration : 2007-05-24
 Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Registration Number : 227MF10163
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2015-06-18
Latest Date of Registration : 2015-06-18
 Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Registration Number : 217MF10438
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2005-08-19
Latest Date of Registration : 2009-11-24
 Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Registration Number : 228MF10141
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2016-08-02
Latest Date of Registration : 2016-08-02
 Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Kewpie is your partner for Sodium Hyaluronate & Hyaluronic Acid// CEP, USDMF, KDMF & JDMF registered.
Registration Number : 217MF10440
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2005-08-19
Latest Date of Registration : 2010-04-01
 HTL Biotechnology is the world leader in the responsible development and production of pharmaceutical-grade biopolymers.
HTL Biotechnology is the world leader in the responsible development and production of pharmaceutical-grade biopolymers.
Purified sodium hyaluronate HMw
Registration Number : 230MF10061
Registrant's Address : 7 rue Alfred Kastler-ZI de l'Aumaillerie-35133 JAVENE-France
Initial Date of Registration : 2018-05-08
Latest Date of Registration : 2018-05-08
 HTL Biotechnology is the world leader in the responsible development and production of pharmaceutical-grade biopolymers.
HTL Biotechnology is the world leader in the responsible development and production of pharmaceutical-grade biopolymers.
Registration Number : 228MF10072
Registrant's Address : 7 rue Alfred Kastler-ZI de l'Aumaillerie-35133 JAVENE-France
Initial Date of Registration : 2016-03-07
Latest Date of Registration : 2023-09-13
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
HyluMed Sterile Sodium Hyaluronate
Registration Number : 220MF10251
Registrant's Address : 500 Kendall Street, Cambridge, Massachusetts 02142, U.S. S. A.
Initial Date of Registration : 2008-12-11
Latest Date of Registration : 2008-12-11
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Registration Number : 219MF10034
Registrant's Address : 50 Binney Street, Cambridge, MA 02142, USA
Initial Date of Registration : 2007-02-05
Latest Date of Registration : 2021-06-15
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Topscience Biotech specializes in R&D, production, and sales of Sodium Hyaluronate, focusing on Medical and Pharmaceutical Grade HA.
Topscience Biotech specializes in R&D, production, and sales of Sodium Hyaluronate, focusing on Medical and Pharmaceutical Grade HA.
Date of Issue : 2022-12-06
Valid Till : 2025-12-18
Written Confirmation Number : SD220042
Address of the Firm : No 98, Lanshan West Road, Lanshan District, Rizhao City, Shandong Province-Post ...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : MFC Corporation
Registration Date : 2019-11-11
Registration Number : 20130322-96-E-109-34(B)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No.678, Tianchen St., High-Tech Development Zone, Jinan, city, Shandong Province Chin...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2022-09-02
Registration Number : 20211021-96-E-171-54(2)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan, Shandong province, China
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : IMCD Korea Co., Ltd.
Registration Date : 2017-12-08
Registration Number : 20151016-96-E-123-38(6)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No. 678 Tianchen St., High-Tech Development Zone, Jian City, Shandong Province, PR of...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2022-03-23
Registration Number : 20130322-96-E-109-34(A)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No. 678 Tianchen St., High-Tech Development Zone, Jian City, Shandong Province, PR of...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Hiple Co., Ltd.
Registration Date : 2022-07-08
Registration Number : 20130322-96-E-109-34(C)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan City, Shandong Province, Chin...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Hiple Co., Ltd.
Registration Date : 2022-07-08
Registration Number : 20211021-96-E-171-54(A)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No. 678 Tianchen Street, High-Tech Development Zone, Jinan, China.
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Seongi Bio Co., Ltd.
Registration Date : 2019-03-06
Registration Number : 20190306-96-E-150-45
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No.678, Tianchen St., High-Tech Development Zone, Jinan, City, Shondong Province, PRo...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Holy Land Ivy Farm
Registration Date : 2017-09-07
Registration Number : 20151016-96-E-123-38(5)
Manufacturer Name : Bloomage Freda Biopharm Co.,...
Manufacturer Address : No.678 Tianchen St., High-Tech Development Zone, Jinan City, Shangdong Province, Chin...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : Korea BMI Co., Ltd.
Registration Date : 2017-09-01
Registration Number : 20130412-96-E-108-34(4)
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No 678, Tianchen St., High-Tech Development Zone, Jinan City, Shandong Province, PR o...
 Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Bloomage is a one-stop source for CEP Sodium Hyaluronate and Hyaluronic Acid// FDA-inspected & GMP-certified.
Registrant Name : MFC Corporation
Registration Date : 2021-10-21
Registration Number : 20211021-96-E-171-54
Manufacturer Name : Bloomage Biotechnology Corp....
Manufacturer Address : No. 678 Tianchen Street, High-Tech Development Zone, Jinan, China.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
16 Oct 2024
Reply
15 Oct 2024
Reply
04 Oct 2024
Reply
04 Oct 2024
Reply
11 Sep 2024
Reply
07 Sep 2024
Reply
13 Aug 2024
Reply
02 Aug 2024
Reply
03 Oct 2023
Reply
08 Aug 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

Sodium Hyaluronate for IR Identification (250...
CAS Number : 9067-32-7
Quantity Per Vial :
Price ($) : 225
Catalog Number : 1614159
Current Lot : F0M296
Previous Lot :
NDC Code :

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?