
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Europe

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
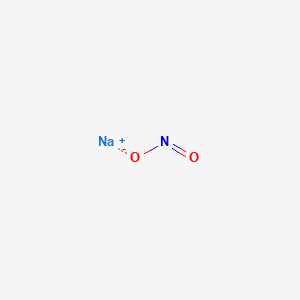

1. Nitrite, Sodium
1. 7632-00-0
2. Nitrous Acid, Sodium Salt
3. Sodium;nitrite
4. Nitrite, Sodium
5. Sodium Nitrite Solution
6. Natrium Nitrit
7. Nitrito Sodico
8. Nitrite De Sodium
9. Natrum Nitrosum
10. Nitrous Acid Soda
11. Nano2
12. Sodium Nitrite [usp]
13. Mfcd00011118
14. Chembl93268
15. Ins No.250
16. M0kg633d4f
17. Chebi:78870
18. Ins-250
19. Nsc-77391
20. Sodium Nitrite (usp)
21. Dsstox_cid_941
22. Dsstox_rid_75879
23. Dsstox_gsid_20941
24. Caswell No. 782
25. Dusitan Sodny [czech]
26. Azotyn Sodowy [polish]
27. Azotyn Sodowy
28. Natrium Nitrit [german]
29. Nitrito Sodico [spanish]
30. Nitrite De Sodium [french]
31. Ccris 559
32. Cas-7632-00-0
33. Hsdb 757
34. Sodium Nitrite Solution, 40 Wt. % In H2o
35. Einecs 231-555-9
36. Nsc 77391
37. Un1500
38. Epa Pesticide Chemical Code 076204
39. Unii-m0kg633d4f
40. Natrium Nitrite
41. Sodium Nitrit
42. Natrii Nitris
43. Nitrous Acid, Sodium Salt (1:1)
44. Sodium Nitrite (tn)
45. Sodium Nitrite Acs Grade
46. Ec 231-555-9
47. Sodium Nitrite [mi]
48. Sodium Nitrite [fcc]
49. Sodium Nitrite [hsdb]
50. Sodium Nitrite [inci]
51. Natrum Nitrosum [hpus]
52. Sodium Nitrite [vandf]
53. Sodium Nitrite [mart.]
54. Sodium Nitrite, Ar, >=98%
55. Sodium Nitrite, Lr, >=98%
56. Dtxsid0020941
57. Sodium Nitrite [usp-rs]
58. Sodium Nitrite [who-dd]
59. Sodium Nitrite [who-ip]
60. Hms3652k08
61. Sodium Nitrite, Analytical Standard
62. Sodium Nitrite, Granular, 99.5%
63. Sodium Nitrite, Trace Metals Grade
64. Tox21_202155
65. Tox21_300025
66. S4074
67. Sodium Nitrite [orange Book]
68. Natrii Nitris [who-ip Latin]
69. Sodium Nitrite [ep Monograph]
70. Akos024427981
71. Sodium Nitrite [usp Monograph]
72. Ccg-266007
73. Ncgc00090737-01
74. Ncgc00090737-02
75. Ncgc00254137-01
76. Ncgc00259704-01
77. Sodium Nitrite [un1500] [oxidizer]
78. Bp-31053
79. E250
80. Nithiodote Component Sodium Nitrite
81. Sodium Nitrite, Acs Reagent, >=97.0%
82. Sodium Nitrite, 0.1m Standardized Solution
83. Sodium Nitrite, P.a., Acs Reagent, 99%
84. Ft-0645124
85. S0565
86. Sodium Nitrite Component Of Nithiodote
87. Sodium Nitrite, 99.5%, Super Free-flowing
88. Sodium Nitrite, Reagentplus(r), >=99.0%
89. Sw219150-1
90. Sodium Nitrite, 99.999% Trace Metals Basis
91. Sodium Nitrite, Saj First Grade, >=97.0%
92. D05865
93. E78844
94. Sodium Nitrite, >=99.99% Trace Metals Basis
95. Sodium Nitrite, Jis Special Grade, >=98.5%
96. Sodium Nitrite, Purum P.a., >=98.0% (rt)
97. Q339975
98. Sodium Nitrite, Puriss. P.a., Acs Reagent, >=99.0% (rt)
99. Sodium Nitrite, United States Pharmacopeia (usp) Reference Standard
100. Nitrite Ion Standard Solution, 0.01 M No2-, For Ion-selective Electrodes
101. Nitrite Ion Standard Solution, 0.1 M No2-, For Ion-selective Electrodes
102. Sodium Nitrite, Anhydrous, Free-flowing, Redi-dri(tm), Acs Reagent, >=97%
103. Sodium Nitrite, Puriss. P.a., Acs Reagent, Reag. Ph. Eur., >=99%
104. Sodium Nitrite, Puriss., Meets Analytical Specification Of Ph. Eur., Bp, Usp, Fcc, E 250, 99-100.5% (calc. To The Dried Substance)
| Molecular Weight | 68.995 g/mol |
|---|---|
| Molecular Formula | NNaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 68.98267252 g/mol |
| Monoisotopic Mass | 68.98267252 g/mol |
| Topological Polar Surface Area | 52.5 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 13.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Sodium nitrite |
| PubMed Health | Sodium Nitrite (Injection) |
| Drug Classes | Cyanide Antidote |
| Drug Label | Sodium nitrite has the chemical name nitrous acid sodium salt. The chemical formula is NaNO2 and the molecular weight is 69.0. The structural formula is:Structure of Sodium NitriteSodium Nitrite Injection is a cyanide antidote which contains one 10 m... |
| Active Ingredient | Sodium nitrite |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 300mg/10ml (30mg/ml) |
| Market Status | Prescription |
| Company | Hope Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Sodium nitrite |
| PubMed Health | Sodium Nitrite (Injection) |
| Drug Classes | Cyanide Antidote |
| Drug Label | Sodium nitrite has the chemical name nitrous acid sodium salt. The chemical formula is NaNO2 and the molecular weight is 69.0. The structural formula is:Structure of Sodium NitriteSodium Nitrite Injection is a cyanide antidote which contains one 10 m... |
| Active Ingredient | Sodium nitrite |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 300mg/10ml (30mg/ml) |
| Market Status | Prescription |
| Company | Hope Pharms |
Antidotes ...
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Has been used as a vasodilator; as a circulatory (blood pressure) depressant and to relieve smooth muscle spasm.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1365
Antidote for cyanide poisoning.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1365
MEDICATION (VET): In cyanide poisoning.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1365
For more Therapeutic Uses (Complete) data for SODIUM NITRITE (12 total), please visit the HSDB record page.
General Toxicity Study: oral human, LDLo 71 mg/kg, coma, gastrointestinal effects, and methemoglobinemia; oral child, LDLo 22 mg/kg, vascular effects.
European Chemicals Bureau; IUCLID Dataset, Sodium nitrite (7632-00-0) (2000 CD-ROM edition). Available from, as of October 27, 2006: https://esis.jrc.ec.europa.eu/
The lethal oral dose of nitrite for adults has been variously reported to be between 0.7 and 6 g NO2- (approximately 10 to 100 mg NO2-/kg). /Nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
Food Preservatives
Substances capable of inhibiting, retarding or arresting the process of fermentation, acidification or other deterioration of foods. (See all compounds classified as Food Preservatives.)
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB08 - Sodium nitrite
In mice given 400, 800, or 1200 mg sodium nitrite orally in drinking water 99.1 to 99.5% of the dose was eliminated. The remaining nitrite was transformed into nitrate & recovered from the liver & muscle.
CANTONI C ET AL; ARCH VET ITAL 32 (1-2): 7 (1981)
Nitrate and nitrite given orally are absorbed and transferred to the blood in the upper part of the gastrointestinal tract. Abundant pectin in the food may delay absorption which may then occur lower down in the intestine, with possible increased risk for microbial transformation of nitrate into nitrite. /Nitrate and nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
Regardless of route of exposure, nitrate and nitrite are rapidly transferred into the blood. Nitrite is gradually oxidized to nitrate which is readily distributed into most body fluids (urine, saliva, gastric juice, sweat, ileostomy fluid). Distribution of nitrate into plasma, erythrocytes, saliva and urine following an oral dose of sodium nitrate has been demonstrated ... /Nitrate and nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
... Transplacental passage of nitrite occurred in pregnant rats given doses at 2.5-50 mg/kg orally ...
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 420
For more Absorption, Distribution and Excretion (Complete) data for SODIUM NITRITE (10 total), please visit the HSDB record page.
... Intestinal bacteria were involved in the reduction of nitrite ... Absorbed nitrite is rapidly oxidized to nitrate in the blood by a mammalian process ... The process of nitrate generation parallels the methemoglobin (MetHb) formation ... Nitrite oxidation to nitrate may also occur in the stomach prior to absorption, as demonstrated in vitro for mice. However, under in vivo conditions, nitrite is probably absorbed from the stomach before large quantities of nitrate are formed. /Nitrite/
WHO; WHO Food Additives Series 35 (844): Nitrite. Available from, as of October 27, 2006: https://www.inchem.org/documents/jecfa/jecmono/v35je13.htm
... Nitrite may be further reduced to nitrogen by bacteria under some conditions. In blood, nitrite transforms hemoglobin to methemoglobin and is simultaneously oxidized to nitrate. Normally methemoglobin gradually reverts to hemoglobin through enzymatic reactions. Nitrite has vasodilating properties, probably through transformation into nitric oxide (NO) or a NO-containing molecule acting as a signal factor for smooth muscle relaxation. Nitrite easily transforms into a nitrosating agent in an acidic environment and can react with a variety of compounds, eg ascorbic acid, amines, amides. Nitrosation can also be mediated by bacteria, eg in the stomach. Some reaction products are carcinogenic (eg most nitrosoamines and amides). /Nitrate and nitrite/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
No or very slight increase in blood nitrosamine level was found in human subjects after consumption of nitrate-, nitrite-, and/or amine-rich meals /Nitrate, nitrite, and amine/
WHO; WHO Food Additives Series 35 (844): Nitrite. Available from, as of October 27, 2006: https://www.inchem.org/documents/jecfa/jecmono/v35je13.htm
The details of nitrite metabolism became a more complex when it was recognized that conversion of nitrite into nitric oxide can occur under certain physiological conditions such as hypoxia. This represents a reversal of the well-known nitric oxide-to-nitrite/nitrate pathways. As noted earlier, experiments in the 1980s showed that the nitrite ion could react with deoxygenated hemoglobin to release nitric oxide, but these experiments were carried out in vitro and their potential physiological relevance was not apparent. Zweier et al. (1995) reported that, in a perfused heart model for ischaemia, nitrite ion was converted in vivo directly into nitric oxide, which demonstrated that the earlier observations did in fact have biochemical implications. These studies have been followed by several related experiments indicating a renewed interest in the biochemistry of nitrite/nitrate. A model for nitrite/nitrate metabolism has emerged based on extensive and sometimes subtle interactions among ingested and endogenously synthesized nitrate, nitrite, nitric oxide and some related species, and the physiology of the organism.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V. 94: p. 272(2010)
There is an active endogenous nitrogen cycle in humans that involves nitrate and nitrite, which are interconvertible in vivo. Nitrosating agents that arise from nitrite under acidic gastric conditions react readily with nitrosatable compounds, especially secondary amines and amides, to generate N-nitroso compounds. These nitrosating conditions are enhanced following ingestion of additional nitrate, nitrite or nitrosatable compounds. Some of the N-nitroso compounds that could be formed in humans under these conditions are known carcinogens.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V. 94: p. 323 (2010)
To clarify the mechanisms underlying forestomach carcinogenesis in rats by co-treatment with catechol and sodium nitrite (NaNO2), /the authors/ investigated the involvement of oxidative stress resulting from reaction of the two compounds. Since generation of semiquinone radical, hydroxyl radical (*OH), and peroxynitrite (ONOO-) arose through the reaction of catechol with NO, we proposed that superoxide resulting from catechol oxidation reacted with excess NO, consequently yielding *OH via ONOO-. Male F344 rats were co-treated with 0.2% catechol in the diet and 0.8% NaNO2 in the drinking water for 2 weeks. Prior to occurrence of histological evidence indicating epithelial injury and hyperplasia, 8-hydroxydeoxyguanosine levels in forestomach epithelium significantly increased from 12 hr together with appearance of immunohistochemically nitrotyrosine-positive epithelial cells. There were no remarkable changes in rats given each chemical alone. We conclude that oxidative stress due to NO plays an important role in induction of forestomach epithelial damage, cell proliferation, and thus presumably forestomach carcinogenesis.
PMID:16530157 Ishii Y et al; Arch Biochem Biophys 447 (2): 127-35 (2006)
... The major concern of possible long-term effects of exposure to nitrate and nitrite is associated with formation of nitroso compounds, many of which are carcinogenic. This formation may take place wherever nitrite and nitrosable compounds are present, but it is favored by acidic conditions or the presence of some bacteria. The gastrointestinal tract and especially the stomach is regarded as the main formation site, but nitrosation reactions can also take place in an infected urinary bladder ... /Nitrate and nitrite poisoning/
IPCS; Poisons Information Monograph G016: Nitrates and nitrites. (September 1996). Available from, as of October 24, 2006: https://www.inchem.org/documents/pims/chemical/pimg016.htm
The two basic actions of sodium nitrite in vivo are relaxation of smooth muscle, especially of small blood vessels, and in toxic doses the conversion of hemoglobin to methemoglobin.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. III-314
For more Mechanism of Action (Complete) data for SODIUM NITRITE (8 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
59
PharmaCompass offers a list of Sodium Nitrite API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Nitrite manufacturer or Sodium Nitrite supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Nitrite manufacturer or Sodium Nitrite supplier.
PharmaCompass also assists you with knowing the Sodium Nitrite API Price utilized in the formulation of products. Sodium Nitrite API Price is not always fixed or binding as the Sodium Nitrite Price is obtained through a variety of data sources. The Sodium Nitrite Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sodium Nitrite manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sodium Nitrite, including repackagers and relabelers. The FDA regulates Sodium Nitrite manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sodium Nitrite API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sodium Nitrite manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sodium Nitrite supplier is an individual or a company that provides Sodium Nitrite active pharmaceutical ingredient (API) or Sodium Nitrite finished formulations upon request. The Sodium Nitrite suppliers may include Sodium Nitrite API manufacturers, exporters, distributors and traders.
click here to find a list of Sodium Nitrite suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sodium Nitrite DMF (Drug Master File) is a document detailing the whole manufacturing process of Sodium Nitrite active pharmaceutical ingredient (API) in detail. Different forms of Sodium Nitrite DMFs exist exist since differing nations have different regulations, such as Sodium Nitrite USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sodium Nitrite DMF submitted to regulatory agencies in the US is known as a USDMF. Sodium Nitrite USDMF includes data on Sodium Nitrite's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sodium Nitrite USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sodium Nitrite suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sodium Nitrite as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sodium Nitrite API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sodium Nitrite as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sodium Nitrite and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sodium Nitrite NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sodium Nitrite suppliers with NDC on PharmaCompass.
Sodium Nitrite Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sodium Nitrite GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sodium Nitrite GMP manufacturer or Sodium Nitrite GMP API supplier for your needs.
A Sodium Nitrite CoA (Certificate of Analysis) is a formal document that attests to Sodium Nitrite's compliance with Sodium Nitrite specifications and serves as a tool for batch-level quality control.
Sodium Nitrite CoA mostly includes findings from lab analyses of a specific batch. For each Sodium Nitrite CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sodium Nitrite may be tested according to a variety of international standards, such as European Pharmacopoeia (Sodium Nitrite EP), Sodium Nitrite JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sodium Nitrite USP).
