
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
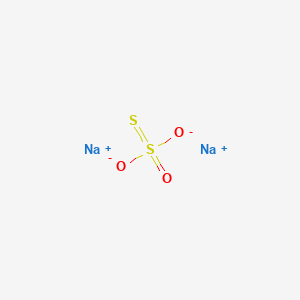

1. Pentahydrate Of Sodium Thiosulfate
2. Sodium Thiosulfate (usan)
3. Sodium Thiosulfate Anhydrous
4. Sodium Thiosulfate Pentahydrate
5. Thiosulfuric Acid, Disodium Salt
1. 7772-98-7
2. Sodiumthiosulfate
3. Disodium Thiosulfate
4. Hypo
5. Chlorine Control
6. Thiosulfuric Acid, Disodium Salt
7. Sodium Thiosulfate, Anhydrous
8. Disodium Sulfurothioate
9. Mfcd00003499
10. Na2s2o3
11. L0iyt1o31n
12. Thiosulfuric Acid (h2s2o3), Disodium Salt
13. Sodothiol
14. Sodium Sulfothioate
15. Sodothiol; Sulfactol; Sulfothiorine
16. Chlorine Cure
17. Declor-it
18. Hypo (van)
19. Hsdb 592
20. Einecs 231-867-5
21. Unii-l0iyt1o31n
22. Sodium Thiosulfate Concentrate
23. Ai3-01237
24. Sodium Thiosulfat
25. Sodium Thio-sulfate
26. Sodium Oxide Sulfide
27. Hypo Alcohol, In Ethanol
28. Anhydrous Sodium Thiosulfate
29. Ec 231-867-5
30. Disodium Thiosulphate
31. Sodium Thiosulfate (anhydrous)
32. Sodium Thiosulfate [mi]
33. Chembl3753202
34. Dtxsid9042417
35. Sodium Thiosulfate [hsdb]
36. Chebi:132112
37. Sodium Thiosulfate Solution, 1 M
38. Sodium Thiosulfate, Ar, >=98%
39. Sodium Thiosulfate, Lr, >=97%
40. Sodium Thiosulfate [who-dd]
41. Sodium Thiosulfate Solution, 0.1 M
42. Sodium Thiosulfate, P.a., 98.0%
43. Akos015856704
44. Akos016372312
45. Sodium Thiosulfate Solution, 0.01 M
46. Sodium Thiosulfate Solution, 0.025 M
47. Sodium Thiosulfate Anhydrous [ii]
48. Bp-21059
49. Sodium Thiosulfate, Reagentplus(r), 99%
50. Ft-0696570
51. O0522
52. Sodium Thiosulfate, 0.1n Volumetric Solution
53. D78333
54. Sodium Thiosulfate, 0.1n Standardized Solution
55. Sodium Thiosulfate, 1.0n Standardized Solution
56. Sodium Thiosulfate, 0.01n Standardized Solution
57. Sodium Thiosulfate, Saj First Grade, >=90.0%
58. Disodium;dioxido-oxo-sulfanylidene-lambda6-sulfane
59. Q339866
60. Sodium Thiosulfate, >=99.99% Trace Metals Basis
61. Sodium Thiosulfate, Vetec(tm) Reagent Grade, 99%
62. Thiosulfuric Acid (h2s2o3), Sodium Salt (1:2)
63. Sodium Thiosulfate Solution, 2 G/dl In Deionized Water
64. Sodium Thiosulfate, Anhydrous, Trace Metals Grade 99.99%
65. Sodium Thiosulfate, Purum P.a., Anhydrous, >=98.0% (rt)
66. Sodium Thiosulfate, Anhydrous, >=98% (calc. To The Dried Substance)
67. Sodium Thiosulfate, Acculute Standard Volumetric Solution, Final Concentration 0.1n
68. Sodium Thiosulfate 2.5m Solution (+/- 0.1m) Ph 7.0 - 9.0 (sodium Carbonate Added As A Preservative)
69. Sodium Thiosulfate Solution, Silver Stain Kit Component, 0.5 % (w/v) Sodium Thiosulfate In H2o
| Molecular Weight | 158.11 g/mol |
|---|---|
| Molecular Formula | Na2O3S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 157.90842477 g/mol |
| Monoisotopic Mass | 157.90842477 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 82.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Sodium thiosulfate |
| PubMed Health | Sodium Thiosulfate (Intravenous) |
| Drug Classes | Cyanide Antidote |
| Drug Label | Sodium thiosulfate has the chemical name thiosulfuric acid, disodium salt, pentahydrate. The chemical formula is Na2S2O3 5H2O and the molecular weight is 248.17. The structural formula is:Structure of Sodium Thiosulfate PentahydrateSodium Thiosulf... |
| Active Ingredient | Sodium thiosulfate |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 12.5gm/50ml (250mg/ml) |
| Market Status | Prescription |
| Company | Hope Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Sodium thiosulfate |
| PubMed Health | Sodium Thiosulfate (Intravenous) |
| Drug Classes | Cyanide Antidote |
| Drug Label | Sodium thiosulfate has the chemical name thiosulfuric acid, disodium salt, pentahydrate. The chemical formula is Na2S2O3 5H2O and the molecular weight is 248.17. The structural formula is:Structure of Sodium Thiosulfate PentahydrateSodium Thiosulf... |
| Active Ingredient | Sodium thiosulfate |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 12.5gm/50ml (250mg/ml) |
| Market Status | Prescription |
| Company | Hope Pharms |
Antidotes; Antitubercular Agents; Antioxidants; Chelating Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
SODIUM THIOSULFATE, USP, IS INTENDED FOR PARENTERAL USE IN TREATMENT OF CYANIDE POISONING, BUT IT MAY BE APPLIED TO SKIN IN VARIOUS FORMS. CUTANEOUS INFECTIONS CAUSED BY STAPH AUREUS & STAPH EPIDERMIDIS MAY RESPOND TO CONCN AS LOW AS 0.5%. CONCN OF 2-8%...USED TO TREAT ACNE; THEY ARE ALSO ANTIFUNGAL.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1005
/SODIUM THIOSULFATE USP/...IS USEFUL IN TOPICAL TREATMENT OF TINEA VERSICOLOR, TINEA CRURIS, & POSSIBLY MOST OTHER DERMATOPHYTOSES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1168
MEDICATION (VET): IN CYANIDE POISONING; HAS BEEN USED...EXTERNALLY IN RINGWORM, MANGE.
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 1122
For more Therapeutic Uses (Complete) data for SODIUM THIOSULFATE (6 total), please visit the HSDB record page.
PHYSICALLY INCOMPATIBLE WITH IV CALCIUM SALTS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 548
3(?). 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-86
Prevention of platinum-induced ototoxic hearing loss
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Antitubercular Agents
Drugs used in the treatment of tuberculosis. They are divided into two main classes: "first-line" agents, those with the greatest efficacy and acceptable degrees of toxicity used successfully in the great majority of cases; and "second-line" drugs used in drug-resistant cases or those in which some other patient-related condition has compromised the effectiveness of primary therapy. (See all compounds classified as Antitubercular Agents.)
POORLY ABSORBED FROM BOWEL. ... THIOSULFATE ION DISTRIBUTES ITSELF IN EXTRACELLULAR FLUID.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-86
/ANTIFUNGAL ACTION OF SODIUM THIOSULFATE, USP,/...IS PROBABLY ATTRIBUTABLE TO SLOW RELEASE OF COLLOIDAL SULFUR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1168
IN CYANIDE POISONING, SODIUM NITRITE IS INJECTED IV...TO PRODUCE METHEMOGLOBIN WHICH COMBINES WITH...CYANIDE ION & RENDERS IT TEMPORARILY INACTIVE IN FORM OF CYANMETHEMOGLOBIN. SODIUM THIOSULFATE /USP/ IS THEN INJECTED IV TO FORM NONTOXIC THIOCYANATE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 779
THIOSULFATE SERVES AS SUBSTRATE FOR ENZYME RHODANESE, WHICH MEDIATES CONVERSION OF CYANIDE TO MUCH LESS TOXIC THIOCYANATE, WHICH IS EXCRETED IN URINE.
Casarett, L.J., and J. Doull. Toxicology: The Basic Science of Poisons. New York: MacMillan Publishing Co., 1975., p. 239

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
80
PharmaCompass offers a list of Sodium Thiosulfate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Thiosulfate manufacturer or Sodium Thiosulfate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Thiosulfate manufacturer or Sodium Thiosulfate supplier.
PharmaCompass also assists you with knowing the Sodium Thiosulfate API Price utilized in the formulation of products. Sodium Thiosulfate API Price is not always fixed or binding as the Sodium Thiosulfate Price is obtained through a variety of data sources. The Sodium Thiosulfate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sodium Thiosulfate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sodium Thiosulfate, including repackagers and relabelers. The FDA regulates Sodium Thiosulfate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sodium Thiosulfate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sodium Thiosulfate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sodium Thiosulfate supplier is an individual or a company that provides Sodium Thiosulfate active pharmaceutical ingredient (API) or Sodium Thiosulfate finished formulations upon request. The Sodium Thiosulfate suppliers may include Sodium Thiosulfate API manufacturers, exporters, distributors and traders.
click here to find a list of Sodium Thiosulfate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sodium Thiosulfate DMF (Drug Master File) is a document detailing the whole manufacturing process of Sodium Thiosulfate active pharmaceutical ingredient (API) in detail. Different forms of Sodium Thiosulfate DMFs exist exist since differing nations have different regulations, such as Sodium Thiosulfate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sodium Thiosulfate DMF submitted to regulatory agencies in the US is known as a USDMF. Sodium Thiosulfate USDMF includes data on Sodium Thiosulfate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sodium Thiosulfate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sodium Thiosulfate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sodium Thiosulfate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sodium Thiosulfate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sodium Thiosulfate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sodium Thiosulfate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sodium Thiosulfate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sodium Thiosulfate suppliers with NDC on PharmaCompass.
Sodium Thiosulfate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sodium Thiosulfate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sodium Thiosulfate GMP manufacturer or Sodium Thiosulfate GMP API supplier for your needs.
A Sodium Thiosulfate CoA (Certificate of Analysis) is a formal document that attests to Sodium Thiosulfate's compliance with Sodium Thiosulfate specifications and serves as a tool for batch-level quality control.
Sodium Thiosulfate CoA mostly includes findings from lab analyses of a specific batch. For each Sodium Thiosulfate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sodium Thiosulfate may be tested according to a variety of international standards, such as European Pharmacopoeia (Sodium Thiosulfate EP), Sodium Thiosulfate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sodium Thiosulfate USP).
