
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
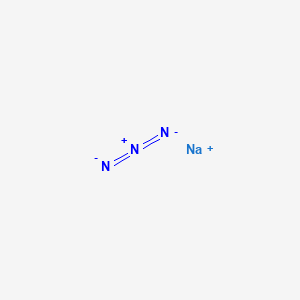

1. Azide, Sodium
2. Nan3
1. 26628-22-8
2. Azide, Sodium
3. Hydrazoic Acid Sodium Salt
4. Natriumazid
5. Sodiumazide
6. Sodium;azide
7. Azoture De Sodium
8. Hydrazoic Acid, Sodium Salt
9. Azidosodium
10. Sodium Trinitride
11. Chembl89295
12. 968jj8c9dv
13. Chebi:278547
14. Nemazyd
15. Dsstox_cid_121
16. Dsstox_rid_75383
17. Dsstox_gsid_20121
18. Natriumazid [german]
19. Natriummazide [dutch]
20. Azydek Sodu [polish]
21. Caswell No. 744a
22. Natriummazide
23. Azydek Sodu
24. Nsc 3072
25. Rcra Waste Number P105
26. Azoture De Sodium [french]
27. Sodium, Azoture De
28. Sodium, Azoturo Di
29. Sodium, Azoture De [french]
30. Sodium, Azoturo Di [italian]
31. Cas-26628-22-8
32. Ccris 1261
33. Hsdb 695
34. Nan3
35. Nci-c06462
36. Einecs 247-852-1
37. Un1687
38. Rcra Waste No. P105
39. Epa Pesticide Chemical Code 107701
40. Unii-968jj8c9dv
41. Azido Sodium
42. Sodium Azid
43. Sodium-azide
44. Natrium Azide
45. Ai3-50436
46. Sodium Azid E
47. U-3886
48. Mfcd00003536
49. Azide, 2% Solution
50. Sodium Azide [mi]
51. Ec 247-852-1
52. Sodium Azide [hsdb]
53. Dtxsid8020121
54. Tox21_202461
55. Tox21_300024
56. Sodium Azide [un1687] [poison]
57. Akos015833396
58. Akos015951264
59. Ncgc00090996-01
60. Ncgc00254054-01
61. Ncgc00260010-01
62. S0489
63. Sodium Azide Solution In Water, 10% Wt/volume
64. Q407577
65. J-016500
| Molecular Weight | 65.010 g/mol |
|---|---|
| Molecular Formula | N3Na |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 64.99899129 g/mol |
| Monoisotopic Mass | 64.99899129 g/mol |
| Topological Polar Surface Area | 3 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 15.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
... The body of a laboratory assistant, was discovered by his colleagues in the laboratory, seated on a chair located near a digital computer displaying information about sodium azide. Moreover, a half empty 99% sodium azide flask was found near the corpse. ... The elevated sodium azide concentration found in the gastric sample and the amount of gastric content allowed to conclude that sodium azide intake was more than 6 g which was above the lethal dose, i.e. approximately 1 g. ...
PMID:22559996 Le Blanc-Louvry I et al; Forensic Sci Int 221 (1-3): e17-20 (2012)
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Mutagens
Chemical agents that increase the rate of genetic mutation by interfering with the function of nucleic acids. A clastogen is a specific mutagen that causes breaks in chromosomes. (See all compounds classified as Mutagens.)
... Sodium azide appeared in rat plasma 5 minutes after a single oral dose at 40 mg/kg and ... by 24 hours, no azide could be detected in either blood or peripheral tissues. A small fraction (7.9 ug) of the administered dose was eliminated in rat urine, but no azide was detected in expired air or feces.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 2-3
When rats were given daily doses of 23 mg/kg sodium azide in their drinking water for 147 days, no azide could be found in their blood.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 3
... For treatment of foot rot /in the bovine hoof/ ... the penetration rate ... of sodium azide /is/ less than 0.05 to 0.24 mm per hr. ... Inclusion of sodium lauryl sulfate in treatments enhanced the penetration rate of ... azide approx 6-fold.
Malecki JC, McCausland IP; Res Vet Sci 3(2): 192-7 (1982)
... Hepatic biotransformation was considered to be the primary route of detoxification based on in vitro data.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 2-3
A case of fatal sodium azide poisoning induced by suicidal ingestion was reported. When the patient arrived, her vital signs such as consciousness and blood pressure, were normal. But 25 hours after ingestion, she died from metabolic acidosis, ARDS (acute respiratory distress syndrome) and acute cardiac failure. We detected the azide ion in patient's serum using GCMS method and measured the blood concentration of sodium azide using the GC/NPD method. The half-life period of sodium azide in blood was calculated as about 2.5 hours.
PMID:11806101 Senda T et al; Chudoku Kenkyu 14 (4): 339-42 (2001)
Sodium azide /is/ an inhibitor of mitochondrial ATPase. ...
Doucet A, Katz AI; Am J Physiol 242 (4): F346-52 (1982)
ABOUT THIS PAGE
33
PharmaCompass offers a list of Sodium Azide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Azide manufacturer or Sodium Azide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Azide manufacturer or Sodium Azide supplier.
PharmaCompass also assists you with knowing the Sodium Azide API Price utilized in the formulation of products. Sodium Azide API Price is not always fixed or binding as the Sodium Azide Price is obtained through a variety of data sources. The Sodium Azide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sodium trinitride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sodium trinitride, including repackagers and relabelers. The FDA regulates Sodium trinitride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sodium trinitride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Sodium trinitride supplier is an individual or a company that provides Sodium trinitride active pharmaceutical ingredient (API) or Sodium trinitride finished formulations upon request. The Sodium trinitride suppliers may include Sodium trinitride API manufacturers, exporters, distributors and traders.
click here to find a list of Sodium trinitride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sodium trinitride DMF (Drug Master File) is a document detailing the whole manufacturing process of Sodium trinitride active pharmaceutical ingredient (API) in detail. Different forms of Sodium trinitride DMFs exist exist since differing nations have different regulations, such as Sodium trinitride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sodium trinitride DMF submitted to regulatory agencies in the US is known as a USDMF. Sodium trinitride USDMF includes data on Sodium trinitride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sodium trinitride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sodium trinitride suppliers with USDMF on PharmaCompass.
Sodium trinitride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sodium trinitride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sodium trinitride GMP manufacturer or Sodium trinitride GMP API supplier for your needs.
A Sodium trinitride CoA (Certificate of Analysis) is a formal document that attests to Sodium trinitride's compliance with Sodium trinitride specifications and serves as a tool for batch-level quality control.
Sodium trinitride CoA mostly includes findings from lab analyses of a specific batch. For each Sodium trinitride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sodium trinitride may be tested according to a variety of international standards, such as European Pharmacopoeia (Sodium trinitride EP), Sodium trinitride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sodium trinitride USP).
