
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
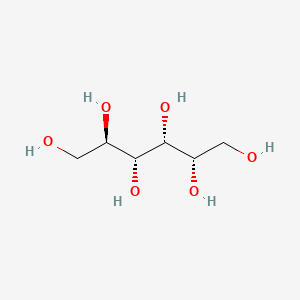

1. Glucitol
2. Klysma Sorbit
3. Medevac
4. Sorbilax
5. Yal
1. D-sorbitol
2. D-glucitol
3. 50-70-4
4. Glucitol
5. L-gulitol
6. (-)-sorbitol
7. Glucarine
8. Diakarmon
9. Multitol
10. Sorbilande
11. Sorbostyl
12. D-(-)-sorbitol
13. Esasorb
14. Neosorb
15. Nivitin
16. Siosan
17. Sorbite
18. Sorbol
19. Cholaxine
20. Sionit
21. Sionite
22. Sionon
23. Sorbo
24. Karion Instant
25. Sorbitol F
26. Sorbex Rp
27. Sorbitol Fp
28. D-sorbol
29. Sionit K
30. Sorbex M
31. Sorbex R
32. Sorbex S
33. Sorbex X
34. Sorbitol Syrup C
35. Hexahydric Alcohol
36. Sorbicolan
37. Sorvilande
38. Gulitol
39. (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexaol
40. Neosorb P 60
41. D-sorbite
42. Foodol D 70
43. Sorbitol Solutions
44. Neosorb 20/60dc
45. Neosorb 70/02
46. Neosorb 70/70
47. Glucitol, D-
48. Neosorb P 20/60
49. Karion
50. D-sorbit
51. Karion (carbohydrate)
52. Fema No. 3029
53. Probilagol
54. (2r,3r,4r,5s)-hexane-1,2,3,4,5,6-hexol
55. D-1,2,3,4,5,6-hexanehexol
56. Ccris 1898
57. G-ol
58. Neosorb P 60w
59. Ai3-19424
60. Hsdb 801
61. Iso-sorbide
62. D-glucitol Syrup
63. Glc-ol
64. Nsc 25944
65. Sorbitol (e420)
66. Chebi:17924
67. D-glucitol, Homopolymer
68. Ins No.420(i)
69. Ins-420(i)
70. Resulax
71. Sorbilax
72. 1,2,3,4,5,6-hexanehexol
73. E 420
74. E-420(i)
75. Nsc-25944
76. 506t60a25r
77. 7b5697n
78. D-sorbit 1000 Microg/ml In Methanol
79. E420
80. Medevac
81. Dsstox_cid_3588
82. Dsstox_rid_77095
83. Dsstox_gsid_23588
84. Sorbitur
85. (2s,3r,4r,5r)-hexane-1,2,3,4,5,6-hexol
86. 26566-34-7
87. Sorbit Dp
88. Cas-50-70-4
89. 123236-29-3
90. Smr000112219
91. Sorbitol [usp:nf]
92. Sorbitol 3% In Plastic Container
93. Wurcs=2.0/1,1,0/[h2122h]/1/
94. Einecs 200-061-5
95. Mfcd00004708
96. Solbitol
97. Sorbitol 3.3% In Plastic Container
98. Sorbitol S
99. Dtxsid5023588
100. Sorbitol Fk
101. Unii-506t60a25r
102. Sorbit D-powder
103. Sorbit S
104. Sorbit W-powder
105. Sorbit Wp
106. Sorbitol (nf)
107. Neosorb P60
108. Sorbitol F Solution
109. Kyowa Powder 50m
110. Sorbogem 712
111. Sorbitol (glucitol)
112. Sorbit D 70
113. Sorbit Dp 50
114. Sorbit L 70
115. Sorbit T 70
116. Sorbit W 70
117. D-sorbitol, 99%
118. Sorbit W-powder 50
119. D-[2-2h]glucitol
120. D-sorbitol; D-glucitol
121. D-sorbitol (jp17)
122. Sorbitol [hsdb]
123. Sorbitol [inci]
124. Sorbitol [fcc]
125. Sorbitol [usp]
126. Sorbitol [ii]
127. Sorbitol [mi]
128. Sorbitol [vandf]
129. Sorbitol Solution (usp)
130. D-sorbitol, >=98%
131. D-sorbitol [jan]
132. Schembl763
133. Sorbit Kyowa Powder 50m
134. Sorbitol [mart.]
135. Bmse000115
136. Bmse000803
137. Bmse001007
138. D-sorbitol [fhfi]
139. Epitope Id:114708
140. Sorbitol [usp-rs]
141. Sorbitol [who-dd]
142. Isomalt Impurity, Sorbitol-
143. D-sorbitol, Nf/fcc Grade
144. Chembl1682
145. Mls001333209
146. Mls001333210
147. Sorbitol [orange Book]
148. D-sorbitol, Analytical Standard
149. D-sorbitol, For Electrophoresis
150. Sorbitol [ep Monograph]
151. D-sorbitol, Bioxtra, >=98%
152. D-sorbitol, For Synthesis, 99%
153. Hms2094k21
154. Hms2270a18
155. Pharmakon1600-01300028
156. Hy-b0400
157. Tox21_201937
158. Tox21_303388
159. D-sorbitol, >=98%, Fcc, Fg
160. Nsc759608
161. S2393
162. Zinc18279893
163. Akos015899604
164. D-sorbitol, Plant Cell Culture Tested
165. Ccg-229392
166. Db01638
167. Nsc-759608
168. Sorbitol 3% In Plastic Container (tn)
169. D-sorbitol Solution, 70% In H2o, Cp
170. Isomalt Impurity C [ep Impurity]
171. Ncgc00164353-01
172. Ncgc00164353-02
173. Ncgc00164353-03
174. Ncgc00257447-01
175. Ncgc00259486-01
176. Ac-13186
177. Cs-13177
178. Maltitol Impurity A [ep Impurity]
179. D-sorbitol, Saj First Grade, >=97.0%
180. Sbi-0206688.p002
181. Sorbitol-mannitol Component Sorbitol
182. D-sorbitol, For Molecular Biology, >=98%
183. D-sorbitol, Bioultra, >=99.5% (hplc)
184. D-sorbitol, Saj Special Grade, >=99.0%
185. D-sorbitol, Vetec(tm) Reagent Grade, 97%
186. E-420
187. S0065
188. Sw220289-1
189. D-sorbitol, Crystallized, >=99.0% (hplc)
190. Sorbitol Component Of Sorbitol-mannitol
191. A15606
192. C00794
193. D00096
194. E70384
195. Ab00919085_06
196. D-sorbitol, Liquid, Tested According To Ph.eur.
197. Isomalt Impurity, Sorbitol- [usp Impurity]
198. Q245280
199. 5-(4-methoxyphenyl)-1,3-oxazole-4-carboxylicacid
200. Lactitol Monohydrate Impurity E [ep Impurity]
201. Mixed With Ethyl Acetate Fraction Of Plinia Cauliflora
202. Mixed With Tannin Enriched Fraction Of Plinia Cauliflora
203. Sorbitol, European Pharmacopoeia (ep) Reference Standard
204. 75de42c3-7c3b-4802-95e0-463f02268bdc
205. Sorbitol, United States Pharmacopeia (usp) Reference Standard
206. D-sorbitol, Bioreagent, Cell Culture Tested, Plant Cell Culture Tested
207. Sorbitol, Pharmaceutical Secondary Standard; Certified Reference Material
208. Sorbitol F Solution, 70 Wt. % In H2o, Contains Mainly D-sorbitol With Lesser Amounts Of Other Hydrogenated Oligosaccharides
| Molecular Weight | 182.17 g/mol |
|---|---|
| Molecular Formula | C6H14O6 |
| XLogP3 | -3.1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 182.07903816 g/mol |
| Monoisotopic Mass | 182.07903816 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 105 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cathartics; Diuretics, Osmotic; Indicators and Reagents; Pharmaceutic Aids
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A polyhydric alcohol with about half the sweetness of sucrose. Sorbitol occurs naturally and is also produced synthetically from glucose. It was formerly used as a diuretic and may still be used as a laxative and in irrigating solutions for some surgical procedures. It is also used in many manufacturing processes, as a pharmaceutical aid, and in several research applications.
National Library of Medicine; 2010 MeSH MeSH Descriptor Data for Sorbitol. Available from, as of March 27, 2010: https://www.nlm.nih.gov/cgi/mesh/2010/MB_cgi?term=Sorbitol
The objective of this report is to describe a cost-effective strategy for management of constipation in nursing home residents with dementia. ... A prospective observational quality improvement study of 41 residents with chronic constipation and receiving an osmotic laxative /was conducted/. Sorbitol was substituted for lactulose. ... The number and amount of laxative use over a period of 4 weeks that were required to maintain regular bowel function was measured. . RESULTS: There was no difference in efficacy of lactulose and sorbitol. Use of additional laxatives was infrequent ... .
Volicer L et al; J Am Med Directors Assoc 6 (3 Supp): 32-4 (2005). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15890292
Osmotic diuretic given iv in 50% (wt/vol) solution to diminish edema, to lower cerebrospinal pressure, or to reduce intraocular pressure in glaucoma ... Dose: 50 to 100 mL of 50% solution; as laxative, oral, 30-50 g. /Former use/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1235
For more Therapeutic Uses (Complete) data for D-Sorbitol (8 total), please visit the HSDB record page.
It is not to be injected. /Sorbitol solution USP/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1235
The administration of a cathartic alone has no role in the management of the poisoned patient and is not recommended as a method of gut decontamination. Experimental data are conflicting regarding the use of cathartics in combination with activated charcoal. No clinical studies have been published to investigate the ability of a cathartic, with or without activated charcoal, to reduce the bioavailability of drugs or to improve the outcome of poisoned patients. Based on available data, the routine use of a cathartic in combination with activated charcoal is not endorsed. If a cathartic is used, it should be limited to a single dose in order to minimize adverse effects.
PMID:9482428 Barceloux D et al; J Toxicol Clin Toxicol 7: 743-52 (1997).
Side effects occur rarely following rectal administration of glycerin or sorbitol ... Rectal discomfort, irritation, burning or griping, cramping pain, and tenesmus /(straining)/. Hyperemia of rectal mucosa with minimal amounts of hemorrhage and mucus discharge ... occur less frequently following rectal administration of sorbitol.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 56:12
Diarrhea frequently occurs with dosages of sorbitol used as adjuncts to sodium polystyrene sulfonate therapy.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 56:12
For more Drug Warnings (Complete) data for D-Sorbitol (15 total), please visit the HSDB record page.
Used as a non-stimulant laxative via an oral suspension or enema.
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
Cathartics
Agents that are used to stimulate evacuation of the bowels. (See all compounds classified as Cathartics.)
Sweetening Agents
Substances that sweeten food, beverages, medications, etc., such as sugar, saccharine or other low-calorie synthetic products. (From Random House Unabridged Dictionary, 2d ed) (See all compounds classified as Sweetening Agents.)
A06AD18
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AD - Osmotically acting laxatives
A06AD18 - Sorbitol
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AG - Enemas
A06AG07 - Sorbitol
B - Blood and blood forming organs
B05 - Blood substitutes and perfusion solutions
B05C - Irrigating solutions
B05CX - Other irrigating solutions
B05CX02 - Sorbitol
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CC - Tests for bile duct patency
V04CC01 - Sorbitol
Route of Elimination
Sorbitol will either be excreted in the urine by the kidneys, or metabolized to carbon dioxide and dextrose.
The amounts of sorbitol (SOR) excreted in 24-hr urine were determined on two groups, ie, diabetic and nondiabetic patients, using an improved method in which ion exchange resin column processing was applied, and these levels were compared with SOR levels in whole blood. Urinary SOR concentration was also determined in diabetic and normal rats in the same manner and its relationship to aldose reductase (AR) activity in whole blood was investigated. Changes in SOR levels in urine and whole blood were compared in diabetic rats after administration of an AR inhibitor (ARI). Whole blood SOR levels and urinary SOR excretion were significantly higher in diabetic patients than in nondiabetic patients. The same results were obtained in the animal models. In diabetic rats, the urinary SOR excretion was about five times higher than that in control rats, and the AR activity in whole blood was also significantly higher. The increase in urinary SOR excretion and whole blood SOR levels, as well as AR activity, in blood in the diabetic state was inhibited by ARI administration. The influence of the diabetic state and the efficacy of the ARI were more marked in urinary SOR excretion than in whole blood SOR levels. These data indicate that determinations of urinary SOR excretion and AR activity are easily measurable and of benefit to assessing the diabetic condition.
Nakano I et al; J Diabet Complic 17 (6): 337-42 (2003). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14583178
An accelerated polyol pathway in diabetes contributes to the development of diabetic complications. To elucidate diabetic nephropathy involving also renal tubular damage, ...urinary sorbitol concentrations /were measured/ concomitantly with urinary N-acetyl-D-glucosaminidase (NAG) excretion in WBN-kob diabetic rats.Twenty-four-hour urinary sorbitol concentrations increased in the diabetic rats in parallel with whole blood sorbitol concentrations. An increase in 24-h urinary NAG excretion coincided with the elevated urinary sorbitol levels in the diabetic rats. The administration of epalrestat, an aldose reductase inhibitor, reduced the increased whole blood and urinary sorbitol concentrations and urinary NAG excretion concomitantly with renal aldose reductase inhibition in the diabetic rats. These results indicate that diabetic nephropathy involves distorted cell function of renal tubules, and that treatment with epalrestat may prevent at least the progress of the nephropathy.
Tsugawa T et al; J Endocrinol 181 (3): 429-35 (2004). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15171691
The purpose of this study was to determine whether sorbitol concentration is elevated in the cerebrospinal fluid (CSF) of non-medically ill patients with mood disorders. Lumbar punctures were performed on 30 subjects - 10 with bipolar mood disorder, 10 with unipolar mood disorder, and 10 age-matched normal controls, and CSF sorbitol concentrations were measured, using a gas chromatographic-mass spectroscopic technique. The mean+/-standard deviation of CSF sorbitol concentrations differed among the three groups as follows: bipolar (22.9+/-4.6 umoles/L) > unipolar (19.0+/-2.8 umoles/L)>normal control (15. 6+/-1.9 umoles/L). One-way ANOVA showed significant (P=0.0002) differences among the three groups. Post-hoc tests indicated a significant (P<0.05) difference between bipolars and normal controls, bipolars and unipolars, and unipolars and normal controls...
Regenold WT et al; Psychoneuroendocrinology 25 (6): 593-606 (2000). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10840171
Streptozocin (Str) diabetic rats were obtained by Str iv (35 mg/kg). Glycemia and sorbitol levels from sciatic nerve and lens were measured after 1 d, 2, 5, and 8 months of diabetes. Sorbitol concentrations in serum, heart, diaphragm, small intestine, and kidney after 8 months of diabetes were measured. RESULTS: Diabetic rats after Str injection showed hyperglycemia (> 1.7 g.L-1), hyperphagia, polyuria, polydipsia, and loss of body weight. Sorbitol levels in lens and sciatic nerve increased in normal and diabetic rats; the increase was higher in diabetic rats. No relationship was shown between glycemia and sorbitol levels. An increased sorbitol level after 8 months of diabetes was found in small intestine and kidney...
Ferraz M et al; Acta Pharmacol Sinica 18 (4): 309-11 (1997). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10072911
For more Absorption, Distribution and Excretion (Complete) data for D-Sorbitol (8 total), please visit the HSDB record page.
Sorbitol is widely used in a number of pharmaceutical products and occurs naturally in many edible fruits and berries. It is absorbed more slowly from the gastrointestinal tract than sucrose and is metabolized in the liver to fructose and glucose ... Sorbitol is better tolerated by diabetics than sucrose and is widely used in many sugar-free liquid vehicles ...
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 681
70% of orally ingested sorbitol is converted to carbon dioxide without appearing as glucose in the blood ...
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1498
Sorbitol exerts its laxative effect by drawing water into the large intestine, thereby stimulating bowel movements.
... Sorbitol exerts hygroscopic and/or local irritant action, drawing water from tissues into feces and reflexly stimulating evacuation.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 56:12
The polyol pathway consists of two enzymes aldose reductase (AR) and sorbitol dehydrogenase (SDH); the former is the first enzyme in the polyol pathway, that catalyzes the reduction of glucose to sorbitol, the latter is the second one, that converts sorbitol to fructose using by NAD(+) as a cofactor. ... SDH activity, the second step in the polyol pathway, might make a greater contribution to the etiology of diabetic retinopathy than does the first step involving AR. /This paper proposes/ a novel hypothesis that polymorphisms of SDH gene may be correlated with SDH gene expression levels in diabetic retinas, thus being a valuable genetic marker for diabetic retinopathy.
Amano S et al; Med Hypothesis 60 (4): 550-1 (2003). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12615520
It has been reported that sorbitol induces apoptosis in several cancer cell lines. ... In /this/ study, the intracellular signaling pathways of sorbitol-induced apoptosis in human K562 cells were investigated using both morphological analysis and DNA fragmentation technique. In this study, we demonstrated that sorbitol-induced apoptosis in human K562 cells is a concentration- and time-dependent manner. This sorbitol-induced apoptosis in human K562 cells was also accompanied by the up-regulation of Bax, and down-regulation of p-Bcl-2, but no effect on the levels of Bcl-X(L). Moreover, the sorbitol treatment resulted in a significant reduction of mitochondria membrane potential, increase in the release of mitochondrial cytochrome c (cyt c), and activation of caspase 3. Furthermore, treatment with caspase 3 inhibitor (z-DEVD-fmk) was capable of preventing the sorbitol-induced caspase 3 activity and cell death. These results clearly demonstrate that the induction of apoptosis by sorbitol involves multiple cellular/molecular pathways and strongly suggest that pro- and anti-apoptotic Bcl-2 family proteins, mitochondrial membrane potential, mitochondrial cyt c, and caspase 3, they all participate in sorbitol-induced apoptotic process in human K562 cells.
Marfe G et al; Arch Toxicol 82 (6): 371-7 (2008). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=18046541
Chronic diabetic complications, in particular, nephropathy, peripheral and autonomic neuropathy, "diabetic foot," retinopathy, and cardiovascular disease, remain the major cause of morbidity and mortality in patients with diabetes mellitus. Growing evidence indicates that both increased activity of the sorbitol pathway of glucose metabolism and enhanced oxidative stress are the leading factors in the pathogenesis of diabetic complications. The relation between the two mechanisms remains the area of controversy. One group has reported that increased sorbitol pathway activity has a protective rather than detrimental role in complication-prone tissues because the pathway detoxifies toxic lipid peroxidation products. Others put forward a so-called "unifying hypothesis" suggesting that activation of several major pathways implicated in diabetic complications (eg, sorbitol pathway) occurs due to increased production of superoxide anion radicals in mitochondria and resulting poly(ADP-ribose) polymerase activation. This review (a) presents findings supporting a key role for the sorbitol pathway in oxidative stress and oxidative stress-initiated downstream mechanisms of diabetic complications, and (b) summarizes experimental evidence against a detoxifying role of the sorbitol pathway, as well as the "unifying concept."
Obrosova IG; Antioxidant Redox Sig 7 (11-12): 1543-52 (2005). Available from, as of March 27, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16356118

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
61
PharmaCompass offers a list of Sorbitol API API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sorbitol API manufacturer or Sorbitol API supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sorbitol API manufacturer or Sorbitol API supplier.
PharmaCompass also assists you with knowing the Sorbitol API API Price utilized in the formulation of products. Sorbitol API API Price is not always fixed or binding as the Sorbitol API Price is obtained through a variety of data sources. The Sorbitol API Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sorbitol API manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sorbitol API, including repackagers and relabelers. The FDA regulates Sorbitol API manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sorbitol API API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sorbitol API manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sorbitol API supplier is an individual or a company that provides Sorbitol API active pharmaceutical ingredient (API) or Sorbitol API finished formulations upon request. The Sorbitol API suppliers may include Sorbitol API API manufacturers, exporters, distributors and traders.
click here to find a list of Sorbitol API suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sorbitol API DMF (Drug Master File) is a document detailing the whole manufacturing process of Sorbitol API active pharmaceutical ingredient (API) in detail. Different forms of Sorbitol API DMFs exist exist since differing nations have different regulations, such as Sorbitol API USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sorbitol API DMF submitted to regulatory agencies in the US is known as a USDMF. Sorbitol API USDMF includes data on Sorbitol API's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sorbitol API USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sorbitol API suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sorbitol API Drug Master File in Japan (Sorbitol API JDMF) empowers Sorbitol API API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sorbitol API JDMF during the approval evaluation for pharmaceutical products. At the time of Sorbitol API JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sorbitol API suppliers with JDMF on PharmaCompass.
Sorbitol API Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sorbitol API GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sorbitol API GMP manufacturer or Sorbitol API GMP API supplier for your needs.
A Sorbitol API CoA (Certificate of Analysis) is a formal document that attests to Sorbitol API's compliance with Sorbitol API specifications and serves as a tool for batch-level quality control.
Sorbitol API CoA mostly includes findings from lab analyses of a specific batch. For each Sorbitol API CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sorbitol API may be tested according to a variety of international standards, such as European Pharmacopoeia (Sorbitol API EP), Sorbitol API JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sorbitol API USP).
