

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
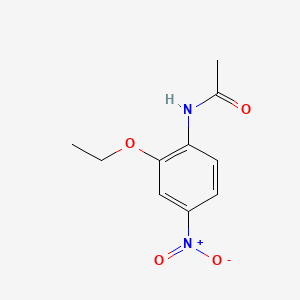

1. 116496-76-5
2. N-(2-ethoxy-4-nitrophenyl)acetamide
3. Acetamide,n-(2-ethoxy-4-nitrophenyl)-
4. 2'-ethoxy-4'-nitroacetanilide
5. Cds1_004703
6. Cbmicro_030367
7. Oprea1_342150
8. Divk1c_005743
9. Schembl2204761
10. Dtxsid60387001
11. Zinc5061120
12. Mfcd00994990
13. Akos001044796
14. Ab07920
15. Ps-6223
16. Bim-0030123.p001
17. Db-049909
18. Ft-0715219
19. Sr-01000223946
20. Sr-01000223946-1
21. 4-(4-ethoxycarbonylthiazol-2-yl)piperazine-1-carboxylic Acid Tert-butyl Ester
| Molecular Weight | 224.21 g/mol |
|---|---|
| Molecular Formula | C10H12N2O4 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 224.07970687 g/mol |
| Monoisotopic Mass | 224.07970687 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 264 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
NDC Package Code : 68225-243
Start Marketing Date : 2024-03-26
End Marketing Date : 2025-12-31
Dosage Form (Strength) : INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION (45mg/mL)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 68225-244
Start Marketing Date : 2024-03-26
End Marketing Date : 2025-12-31
Dosage Form (Strength) : INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION (60mg/mL)
Marketing Category : DRUG FOR FURTHER PROCESSING

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Winrevair (sotatercept) is the first activin signaling inhibitor therapy, which is approved for the treatment of adults with pulmonary arterial hypertension.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Winrevair
Study Phase: ApprovedProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Merck's WINREVAIR™ Approved by European Commission for Pulmonary Hypertension
Details : Winrevair (sotatercept) is the first activin signaling inhibitor therapy, which is approved for the treatment of adults with pulmonary arterial hypertension.
Brand Name : Winrevair
Molecule Type : Large molecule
Upfront Cash : Not Applicable
August 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Winrevair (sotatercept-csrk) is the first activin signaling inhibitor therapy, which is approved for the treatment of adults with pulmonary arterial hypertension.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Winrevair
Study Phase: ApprovedProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Merck Receives Positive EU CHMP Opinion for WINREVAIR™ (sotatercept) in Pulmonary Arterial Hyper...
Details : Winrevair (sotatercept-csrk) is the first activin signaling inhibitor therapy, which is approved for the treatment of adults with pulmonary arterial hypertension.
Brand Name : Winrevair
Molecule Type : Large molecule
Upfront Cash : Not Applicable
June 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Winrevair (sotatercept-csrk) is the first activin signaling inhibitor therapy, which is approved for the treatment of adults with pulmonary arterial hypertension.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Winrevair
Study Phase: ApprovedProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
FDA Approves Merck’s WINREVAIR™ (sotatercept-csrk), a First-in-Class Treatment for Adults with...
Details : Winrevair (sotatercept-csrk) is the first activin signaling inhibitor therapy, which is approved for the treatment of adults with pulmonary arterial hypertension.
Brand Name : Winrevair
Molecule Type : Large molecule
Upfront Cash : Not Applicable
March 26, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MK-7962 (sotatercept) is a potential first-in-class activin signaling inhibitor biologic being studied for the treatment of adult patients with pulmonary arterial hypertension.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: MK-7962
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MK-7962 (sotatercept) is a potential first-in-class activin signaling inhibitor biologic being studied for the treatment of adult patients with pulmonary arterial hypertension.
Brand Name : MK-7962
Molecule Type : Large molecule
Upfront Cash : Not Applicable
September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MK-7962 (sotatercept), is an activin receptor type IIA fusion protein that acts as a ligand trap for members in the transforming growth factor-beta protein superfamily involved in remodeling and regeneration of a variety of different tissues like vasculature and fibrosis.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: MK-7962
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Merck’s Investigational Activin Signaling Inhibitor Sotatercept Improved Six-Minute Walk Distanc...
Details : MK-7962 (sotatercept), is an activin receptor type IIA fusion protein that acts as a ligand trap for members in the transforming growth factor-beta protein superfamily involved in remodeling and regeneration of a variety of different tissues like vascula...
Brand Name : MK-7962
Molecule Type : Large molecule
Upfront Cash : Not Applicable
March 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sotatercept,is an investigational, potential first-in-class activin receptor type IIA-Fc fusion protein demonstrated significant improvement in exercise capacity and key secondary outcome measures compared to placebo when added to background therapy.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: MK-7962
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Sotatercept,is an investigational, potential first-in-class activin receptor type IIA-Fc fusion protein demonstrated significant improvement in exercise capacity and key secondary outcome measures compared to placebo when added to background therapy.
Brand Name : MK-7962
Molecule Type : Large molecule
Upfront Cash : Not Applicable
October 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the successful completion of the acquisition of Acceleron Pharma, Merck would be growing its cardiovascular portfolio and pipeline, including Acceleron’s lead therapeutic candidate, sotatercept, has a novel mechanism of action with the potential to improve short-term.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: ACE-011
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Merck
Deal Size: $11,500.0 million Upfront Cash: $11,500.0 million
Deal Type: Acquisition November 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Merck
Deal Size : $11,500.0 million
Deal Type : Acquisition
Merck Completes Acquisition of Acceleron Pharma Inc.
Details : Through the successful completion of the acquisition of Acceleron Pharma, Merck would be growing its cardiovascular portfolio and pipeline, including Acceleron’s lead therapeutic candidate, sotatercept, has a novel mechanism of action with the potentia...
Brand Name : ACE-011
Molecule Type : Large molecule
Upfront Cash : $11,500.0 million
November 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the acquisition agreement, Merck will acquire all outstanding shares of Acceleron including its pipeline having lead therapeutic candidate, sotatercept, in Phase 3 trials as add-on to current standard of care for the treatment of PAH.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: ACE-011
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Merck & Co
Deal Size: $11,500.0 million Upfront Cash: $11,500.0 million
Deal Type: Acquisition September 30, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : $11,500.0 million
Deal Type : Acquisition
Merck to Acquire Acceleron Pharma Inc
Details : Under the terms of the acquisition agreement, Merck will acquire all outstanding shares of Acceleron including its pipeline having lead therapeutic candidate, sotatercept, in Phase 3 trials as add-on to current standard of care for the treatment of PAH.
Brand Name : ACE-011
Molecule Type : Large molecule
Upfront Cash : $11,500.0 million
September 30, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The SPECTRA Phase 2 trial is a single arm, open-label, multi-center exploratory study to determine the eects of sotatercept plus standard of care in adults with WHO functional class III PAH.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: ACE-011
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : The SPECTRA Phase 2 trial is a single arm, open-label, multi-center exploratory study to determine the eects of sotatercept plus standard of care in adults with WHO functional class III PAH.
Brand Name : ACE-011
Molecule Type : Large molecule
Upfront Cash : Not Applicable
May 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
A total of 106 patients were randomized in a 3:3:4 ratio to receive placebo, sotatercept 0.3 mg/kg, or sotatercept 0.7 mg/kg subcutaneously every three weeks on top of standard-of-care therapies.
Lead Product(s): Sotatercept
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: ACE-011
Study Phase: Phase IIIProduct Type: Large molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sotatercept
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : A total of 106 patients were randomized in a 3:3:4 ratio to receive placebo, sotatercept 0.3 mg/kg, or sotatercept 0.7 mg/kg subcutaneously every three weeks on top of standard-of-care therapies.
Brand Name : ACE-011
Molecule Type : Large molecule
Upfront Cash : Not Applicable
May 19, 2021

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
