
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
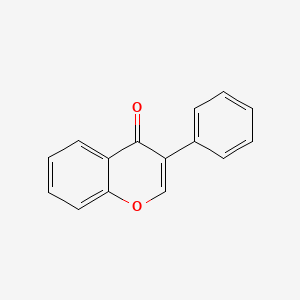

1. 3 Benzylchroman 4 One
2. 3 Benzylchroman 4 Ones
3. 3 Benzylidene 4 Chromanone
4. 3 Benzylidene 4 Chromanones
5. 3-benzylchroman-4-one
6. 3-benzylchroman-4-ones
7. 3-benzylidene-4-chromanone
8. 3-benzylidene-4-chromanones
9. Derivative, Isoflavone
10. Derivatives, Isoflavone
11. Homoisoflavone
12. Homoisoflavones
13. Isoflavone Derivative
14. Isoflavone Derivatives
15. Isoflavones
1. 574-12-9
2. 3-phenyl-4h-chromen-4-one
3. 3-phenylchromen-4-one
4. 4h-1-benzopyran-4-one, 3-phenyl-
5. Isoflavon
6. 3-phenylchromone
7. 3-phenyl-4h-1-benzopyran-4-one
8. Ovo2kuw8h8
9. Nsc-135405
10. Chebi:18220
11. Nsc135405
12. Unii-ovo2kuw8h8
13. Isoflavone - 98%
14. Nsc 135405
15. Isoflavone [mi]
16. Schembl8028
17. Chembl366460
18. Dtxsid90205986
19. Zinc895390
20. Amy33371
21. Bcp22856
22. Mfcd00100851
23. Akos015918505
24. Bcp9000133
25. Ccg-214095
26. Cs-w006405
27. Db12007
28. Ds-6374
29. Hy-w006405
30. Ac-12802
31. Ft-0627415
32. C00799
33. 574i129
34. J-521538
35. Q27102918
36. B-d-glucopyranoside,phenyl2,3-bis-o-(phenylmethyl)-4,6-o-[(r)-phenylmethylene]-1-thio-
| Molecular Weight | 222.24 g/mol |
|---|---|
| Molecular Formula | C15H10O2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 222.068079557 g/mol |
| Monoisotopic Mass | 222.068079557 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 326 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for over-the-counter use as a dietary supplement for increasing bone density and regulating blood fat.
Isolated soy protein with isoflavones was shown to decrease LDL cholesterol levels in randomized trials assessed by the American Heart Association. In a study of postmenopausal women, daily dietary intake of 101 mg of aglycone isoflavones (indicating [DB01645] and [DB13182]) was associated with lowered LDL cholesterol and apolipoprotein B levels by 8% and reduced systolic and diastolic blood pressure by 6.8% in hypertensive women. In a meta-analysis of randomized controlled trials of menopausal women, soy isoflavones attenuated bone loss of the spine and decreased the levels of deoxypyridinoline, a bone resorption marker, while increasing serum bone-specific alkaline phosphatase, a bone formation marker. The findings from studies investigating the effects of soy consumption on menopausal symptoms, breast cancer, and prostate cancer remain somewhat controversial and inconclusive. Consumption of soy isoflavones may decrease the markers of cancer development and progression in prostate cells, including prostate-specific antigen (PSA), testosterone, and androgen receptor in patients with prostate cancer but not in normal subjects. Although epidemiologic data in Asian women demonstrate that high soy food intake is associated with protection against breast cancer, soy foods have little effect on intermediary markers of breast cancer risk and postmenopausal soy intake may not reduce the risk of developing breast cancer. However, preliminary studies show that soy food intake reduces tumor recurrence in breast cancer patients. Soy isoflavones reported to interfere with thyroid peroxidase, which are involved in the production of thyroid hormones.
Absorption
Following oral ingestion, serum isoflavone concentrations increase in a dose-dependent manner. Isoflavones are metabolized by gut microflora, where they need to undergo deglycosylation in order to be absorbed in the intestine. After oral ingestion, glycosylated isoflavones are rapidly deglycosylated, absorbed and metabolized in intestinal enterocytes and liver, entering the systemic circulation predominantly as conjugates with limited bioavailability. In humans, the mean time to reach peak plasma concentrations (Tmax) for conjugated and unconjugated genistein and daidzein are approximately 5-6 and 6-8 hours, respectively.
Route of Elimination
Renal excretion is the predominant route of elimination for dietary isoflavones, where approximately 10-60% of total administered dose is excreted in urine. Glucuronide conjugates account for the majority (70-90%) of the isoflavone content in urine, followed by sulphate conjugates (10-25%) and aglycone forms (1-10%). Fecal excretion is minimal, which accounts for 1-4% of the dietary isoflavone ingested.
Volume of Distribution
Isoflavones are readily distributed to all tissues, and they are known to cross the placental barrier and blood brain barrier. They are also distributed to the extra-vascular compartments. In a human study, the volume of distribution of daidzein and genistein were 336.25 L and 258.76 L, respectively.
Clearance
In a human study, the clearance rate for daidzein and genistein were 30.09 L/h and 21.85 L/h, respectively.
The conversion of glycosylated isoflavones to de glycosylated isoflavones begins in the oral cavity, wherein oral microflora and oral epithelium exhibit -glucosidase activity. Further conversion is mediated by intestinal lactase phlorizin hydrolase on the luminal side of the intestinal brush border to form aglycones that diffuses into the enterocytes. The glycosylated isoflavones may also be converted to aglycone in the large intestines by the resident intestinal microflora. Isoflavone aglycones that enter the intestinal cell via passive diffusion are rapidly conjugated into sulfate or glucuronide conjugates. Under the anaerobic, reductive conditions of the colon, genistein undergoes reduction to form dihydrogenistein and further to 5-hydroxyequol, while daidzein is reduced to dihydrodaidzein and equol. Microbial cleavage of the Ring-C of isoflavones produces deoxybenzoin metabolites (DOBs), which retains similar biological activity as unchanged isoflavones and are passively absorbed. There is a large interindividual variation in isoflavone metabolism, leading to circulating concentrations of isoflavone metabolites and parent isoflavones varying up to hundreds-fold. About 25% of the non-Asian and 50% of the Asian population host the intestinal bacteria that convert the daidzein into the isoflavonoid equol, which is a beneficial isoflavonoid.
The half-life of isoflavones is between 4 and 8 h. Daidzein has a longer intestinal half-life than genistein due to more rapid degradation of genistein. Individual half-life of daidzein and genistein in a human pharmacokinetic study were 7.75 h and 7.77 h, respectively.
Isoflavones are selective estrogen receptor modulators that exert estrogenic-like effects under certain experimental conditions, as they are structurally similar to mammalian 17-estradiol. They may bind to both and isoforms of estrogen receptor (ER), but with binding affinities to ER approximately 20 times higher than that to ER. The role of isoflavones on estrogen-dependent cancer has been studied, since they may mediate antiestrogenic actions by blocking the binding of endogenous estrogens and their receptor signalling. In cell culture, [DB01645] inhibited the proliferation of MDA-MB-231 human breast cancer cells, probably by arresting the cell cycle progression at the G2M transition. In addition, genistein was shown to induce apoptosis, modify eicosanoid metabolism, and inhibit angiogenesis. There is an evidence that soy isoflavones may act on androgen receptors to inhibit tyrosine kinase activity, thereby blocking the growth and proliferation of cancer cells. Isoflavones may not significantly contribute to the hypolipidemic effects of soy protein, but may exert coronary benefits by improving endothelial function; in clinical trials of postmenopausal women, isoflavones improved flow-mediated dilation in women with impaired endothelial function. Some observational data suggests that isoflavones improve endothelial function by increasing the number of circulating endothelial progenitor cells, which replace damaged endothelial cells. Isoflavone may modulate the key transcription factors involved in the regulation of lipid metabolism by acting on the peroxisome proliferator-activated receptors (PPAR) alpha and gamma, which are receptors that regulate the transcription of genes involved in lipid and glucose homeostasis and lipid metabolism. Multiple biological actions of isoflavones, such as favorable effect on the blood lipid profile and inhibition of LDL cholesterol oxidation, may lead to cardio protective effects. [DB01645] has been shown to have antioxidant properties on hydrogen peroxide production _in vitro_ and blocks the formation of oxygen free radicals. Studies also suggest that at micromolar concentrations, genistein increases glucose-stimulated insulin secretion in cell lines and mouse pancreatic islets via a cAMP-dependent protein kinase mechanism. Based on the findings of experimental studies, genistein may exert a positive effect on bone formation by decreasing osteoclastic resorption factor, such as collagen C-telopeptide, and increasing osteoblastic formation markers, such as bone-alkaline phosphatase. _In vitro_, it antagonized the catabolic effects of parathyroid hormone (PTH) in osteoblasts by reversing the PTH-induced increase in soluble receptor activator of nuclear factor-xB ligand and decrease in osteoprotegerin expression.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
ABOUT THIS PAGE
94
PharmaCompass offers a list of Soybean Extract API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Soybean Extract manufacturer or Soybean Extract supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Soybean Extract manufacturer or Soybean Extract supplier.
PharmaCompass also assists you with knowing the Soybean Extract API Price utilized in the formulation of products. Soybean Extract API Price is not always fixed or binding as the Soybean Extract Price is obtained through a variety of data sources. The Soybean Extract Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Soybean Extract manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Soybean Extract, including repackagers and relabelers. The FDA regulates Soybean Extract manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Soybean Extract API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Soybean Extract manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Soybean Extract supplier is an individual or a company that provides Soybean Extract active pharmaceutical ingredient (API) or Soybean Extract finished formulations upon request. The Soybean Extract suppliers may include Soybean Extract API manufacturers, exporters, distributors and traders.
click here to find a list of Soybean Extract suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Soybean Extract DMF (Drug Master File) is a document detailing the whole manufacturing process of Soybean Extract active pharmaceutical ingredient (API) in detail. Different forms of Soybean Extract DMFs exist exist since differing nations have different regulations, such as Soybean Extract USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Soybean Extract DMF submitted to regulatory agencies in the US is known as a USDMF. Soybean Extract USDMF includes data on Soybean Extract's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Soybean Extract USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Soybean Extract suppliers with USDMF on PharmaCompass.
Soybean Extract Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Soybean Extract GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Soybean Extract GMP manufacturer or Soybean Extract GMP API supplier for your needs.
A Soybean Extract CoA (Certificate of Analysis) is a formal document that attests to Soybean Extract's compliance with Soybean Extract specifications and serves as a tool for batch-level quality control.
Soybean Extract CoA mostly includes findings from lab analyses of a specific batch. For each Soybean Extract CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Soybean Extract may be tested according to a variety of international standards, such as European Pharmacopoeia (Soybean Extract EP), Soybean Extract JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Soybean Extract USP).
