
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
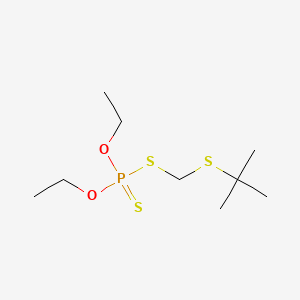

1. O,o-diethyl S((tert-butylthio)methyl)phosphorodithioate
1. Counter
2. 13071-79-9
3. Contraven
4. Aragran
5. Counter 15g Soil Insecticide
6. Ac 92100
7. Ent 27920
8. Counter 15g
9. Counter 15g Soil Insecticide-nematicide
10. S-tert-butylthiomethyl O,o-diethyl Phosphorodithioate
11. Phosphorodithioic Acid S-((tert-butylthio)methyl) O,o-diethyl Ester
12. Phosphorodithioic Acid, O,o-diethyl S-(((1,1-dimethylethyl)thio)methyl) Ester
13. Phosphorodithioic Acid, S-[[(1,1-dimethylethyl)thio]methyl] O,o-diethyl Ester
14. M83bn0f8r9
15. Chebi:38960
16. S-(((1,1-dimethylethyl)thio)methyl) O,o-diethyl Phosphorodithioate
17. S-(((1,1-dimethylethyl)thio)methyl)-o,o-diethyl Phosphorodithioate
18. S-t-butylthio-methyl-o,o-diethyl Phosphorodithioate
19. S-[(tert-butylsulfanyl)methyl] O,o-diethyl Phosphorodithioate
20. Dsstox_cid_2254
21. Phosphorodithioic Acid, S-[(tert-butylthio)methyl] O,o-diethyl Ester
22. Dsstox_rid_76530
23. Dsstox_gsid_22254
24. O,o-diethyl S-(tert-butylthio)methyl Phosphorodithioate
25. S-[(tert-butylthio)methyl] O,o-diethyl Dithiophosphate
26. S-[(tert-butylsulfanyl)methyl] O,o-diethyl Dithiophosphate
27. Caswell No. 131a
28. O,o-diethyl S-(((1,1-dimethylethyl)thio)methyl)phoshorodithioate
29. Phosphorodithioic Acid, S-(((1,1-dimethylethyl)thio)methyl) O,o-diethyl Ester
30. Terbufos [ansi:bsi:iso]
31. Terbufos [iso]
32. Cas-13071-79-9
33. Ccris 4772
34. Hsdb 6444
35. St-100
36. Ai3-27920
37. Einecs 235-963-8
38. Epa Pesticide Chemical Code 105001
39. Brn 1710115
40. S-tert-butylthiomethyl O,o-diethylphosphorodithioate
41. Unii-m83bn0f8r9
42. Cyanater
43. Tert-butylsulfanylmethylsulfanyl-diethoxy-sulfanylidene-?^{5}-phosphane
44. S-((tert-butylthio)methyl)o,o-diethylphosphorodithioate
45. Terbufos [hsdb]
46. Terbufos [mi]
47. Phosphorodithioic Acid S-(((1,1-dimethylethyl)thio)methyl) O,o-diethyl Ester
48. Schembl23773
49. 4-01-00-03092 (beilstein Handbook Reference)
50. Chembl1406292
51. Dtxsid2022254
52. Tox21_201634
53. Tox21_302994
54. Terbufos 10 Microg/ml In Cyclohexane
55. Terbufos 1000 Microg/ml In Acetone
56. Akos016014230
57. Terbufos 10 Microg/ml In Acetonitrile
58. Terbufos 100 Microg/ml In Acetonitrile
59. Ncgc00091771-01
60. Ncgc00091771-02
61. Ncgc00091771-03
62. Ncgc00256426-01
63. Ncgc00259183-01
64. Ac-92100
65. O,o-diethyl-s-1,1-dimethylethylthiomethyl
66. Terbufos, Pestanal(r), Analytical Standard
67. C18693
68. 071t799
69. J-005860
70. Q2404344
71. S-[(tert-butylthio)methyl] O,o-diethyl Phosphorodithioate
72. S-[(tert-butylsulfanyl)methyl] O,o-diethyl Dithiophosphate #
73. O,o-diethyl S-[[(1,1-dimethylethyl)thio]methyl] Phosphorodithioate
74. S-[[(1,1-dimethylethyl)thio]methyl] O,o-diethyl Phoshporodithioate
75. Diethyl {[(tert-butylsulfanyl)methyl]sulfanyl}(sulfanylidene)phosphonite
76. Tert-butylsulfanylmethylsulfanyl-diethoxy-sulfanylidene-lambda5-phosphane
77. Methanethiol, (tert-butylthio)-, S-ester With O,o-diethyl Phosphorodithioate
| Molecular Weight | 288.4 g/mol |
|---|---|
| Molecular Formula | C9H21O2PS3 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 101 |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 206 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A metabolism study of rats indicated that 83% of a single administration of 0.8 mg/kg terbufos was excreted in the urine in the form of metabolites and 3.5% in the feces during 168 hours. No terbufos accumulated in tissues.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 924
Rapidly absorbed through skin.
Farm Chemicals Handbook 2002. Meister Publishing Co., 2002., p. C-384
Among 11 farmers who applied a formulated terbufos product while planting corn, ...no alkyl phosphates were detected in urine... /after an estimated respiratory dose of 0.009 mg/cu m terbufos/.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 924
Metabolism occurs principally by oxidation, hydrolysis by esterases, and by transfer of portions of the molecule to glutathione. Oxidation of organophosphorus insecticides may result in more or less toxic products. The glutathione transferase reactions produce products, that are, in most cases, of low toxicity. Hydrolytic and transferase reactions affect both thioates and their oxons. /Organophosphorus Pesticides/
WHO; Environ Health Criteria 63: Organophosphorus pesticides (1986). Available from, as of July 3,2003: https://www.inchem.org/pages/ehc.html
Biotransformation of terbufos in rat liver revealed four metabolites in the effluent prepared with a C18 cartridge after the rat liver was perfused for one hour in situ. Analysing the spectrogram of GC-IR and GC-MS, metabolite IV appeared to be an oxidative desulfuration product of terbufos with the formula C9H21O3PS2, the recovery of which in the effluent was 2.13%; metabolite I appeared to be an hydrolysate of metabolite IV with the formula C5H13O3PS, the recovery of which was 0.13%; metabolite II appeared to be an hydrolysate of terbufos with the formula C5H13O2PS2, the recovery of which was 2.65%; metabolite III appeared to be a methylate of metabolite II, with the formula C6H15O2PS2, the recovery of which was 1.42%. Relatively the recovery of terbufos was 40.8%. These results were in accord with the regular metabolic pattern in vivo of phosphorothioates with a thioether group.
Li J-T et al; JOURNAL OF OCCUPATIONAL HEALTH 41(2): 62-68 (1999)
The organophosphorus compounds owe their biological activities to the capacity of the central P atom to phosphorylate the esteratic site of the enzyme, cholinesterase, which is an essential constituent of the nervous system not only of Insecta but also of all higher animals. /Organophosphorus compounds/
Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. 13(81) 448
Organophosphates poison insects and mammals primarily by phosphorylation of the acetylcholinesterase enzyme at nerve endings. ... At sufficient dosage, loss of enzyme function allows accumulation of acetylcholine (the impulse- transmitter substance) at cholinergic neuroeffector junctions (muscarinic effects, and at skeletal myoneural junctions and in autonomic ganglia (nicotinic effects). Organophosphates also impair nerve impulse transmission in the brain ... . /Organophosphate pesticides/
U.S. Environmental Protection Agency/Office of Prevention, Pesticides, and Toxic Substances. Reigart, J.R., Roberts, J.R. Recognition and Management of Pesticide Poisonings. 5th ed. 1999. EPA Document No. EPA 735-R-98-003, and available in electronic format at: https://www.epa.gov/pesticides/safety/healthcare, p. 34
ABOUT THIS PAGE
72
PharmaCompass offers a list of ST-100 API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right ST-100 manufacturer or ST-100 supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred ST-100 manufacturer or ST-100 supplier.
PharmaCompass also assists you with knowing the ST-100 API Price utilized in the formulation of products. ST-100 API Price is not always fixed or binding as the ST-100 Price is obtained through a variety of data sources. The ST-100 Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A ST-100 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of ST-100 , including repackagers and relabelers. The FDA regulates ST-100 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. ST-100 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A ST-100 supplier is an individual or a company that provides ST-100 active pharmaceutical ingredient (API) or ST-100 finished formulations upon request. The ST-100 suppliers may include ST-100 API manufacturers, exporters, distributors and traders.
ST-100 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of ST-100 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right ST-100 GMP manufacturer or ST-100 GMP API supplier for your needs.
A ST-100 CoA (Certificate of Analysis) is a formal document that attests to ST-100 's compliance with ST-100 specifications and serves as a tool for batch-level quality control.
ST-100 CoA mostly includes findings from lab analyses of a specific batch. For each ST-100 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
ST-100 may be tested according to a variety of international standards, such as European Pharmacopoeia (ST-100 EP), ST-100 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (ST-100 USP).
