
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
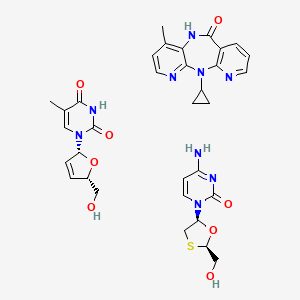

1. Stavudine - Lamivudine - Nevirapine
2. Stavudine, Lamivudine, Nevirapine Drug Combination
3. Triomune
1. 811471-91-7
2. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one;2-cyclopropyl-7-methyl-2,4,9,15-tetrazatricyclo[9.4.0.03,8]pentadeca-1(11),3,5,7,12,14-hexaen-10-one;1-[(2r,5s)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione
3. Lamivudine; Stavudine; Nevirapine
4. Lamivudine/nevirapine/stavudine
5. Stavudine, Lamivudine And Nevirapine
6. Lamivudine Mixture With Nevirapine And Stavudine
7. Stv & Lmv & Nvp
8. Lamivudine, Nevirapine, And Stavudine Fixed-dose Tablet
9. Nevirapine & Lamivudine & Stavudine
10. 11-cyclopropyl-4-methyl-5,11-dihydro-6h-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one & (-)-(2'r,5's)-1-[2'-hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine & Thymidine, 2',3'-didehydro-, 3'-deoxy-
11. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; 11-cyclopropyl-4-methyl-5h-dipyrido[[?],[?]][1,4]diazepin-6-one; 1-[(2r,5s)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione
| Molecular Weight | 719.8 g/mol |
|---|---|
| Molecular Formula | C33H37N9O8S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 5 |
| Exact Mass | 719.24858035 g/mol |
| Monoisotopic Mass | 719.24858035 g/mol |
| Topological Polar Surface Area | 250 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Lamivudine; stavudine; nevirapine |
| Active Ingredient | stavudine; Lamivudine; nevirapine |
| Dosage Form | Tablet; Tablet, dispersible |
| Route | oral |
| Strength | 100mg; 6mg; 30mg; 200mg; 150mg; 12mg; 50mg; 30mg; 40mg; 60mg |
| Market Status | Tentative Approval |
| Company | Cipla |
| 2 of 2 | |
|---|---|
| Drug Name | Lamivudine; stavudine; nevirapine |
| Active Ingredient | stavudine; Lamivudine; nevirapine |
| Dosage Form | Tablet; Tablet, dispersible |
| Route | oral |
| Strength | 100mg; 6mg; 30mg; 200mg; 150mg; 12mg; 50mg; 30mg; 40mg; 60mg |
| Market Status | Tentative Approval |
| Company | Cipla |
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AR - Antivirals for treatment of hiv infections, combinations
J05AR07 - Stavudine, lamivudine and nevirapine
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?