



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
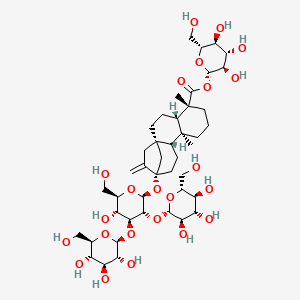

1. 19-o-beta-glucopyranosyl-13-o-(beta-glucopyranosyl(1-2)-beta-glucopyranosyl(1-3))-beta-glucopyranosyl-13-hydroxykaur-16-en-19-oic Acid
2. 19-o-beta-glucopyranosyl-13-o-(beta-glucopyranosyl(1-2)-beta-glucopyranosyl(1-3))-beta-glucopyranosylsteviol
1. 58543-16-1
2. Stevioside A3
3. Rebaudioside-a
4. Truvia
5. Sweetener 4g-s
6. Rebiana
7. Rebaudiana A
8. Sooolite!-pure
9. Reb-a 97
10. Reb A
11. Reb-a
12. Rebaudiosidea
13. B3fud0528f
14. 19-o-beta-glucopyranosyl-13-o-(beta-glucopyranosyl(1-2)-beta-glucopyranosyl(1-3))-beta-glucopyranosyl-13-hydroxykaur-16-en-19-oic Acid
15. 19-o-beta-glucopyranosyl-13-o-(beta-glucopyranosyl(1-2)-beta-glucopyranosyl(1-3))-beta-glucopyranosylsteviol
16. Stevia Powder
17. Stevia
18. Pure Via
19. Glycoside A3
20. Chrysanta Ar-p
21. Rebaudioside A [mi]
22. Unii-b3fud0528f
23. Rebaudioside A [fcc]
24. Rebaudioside A [inci]
25. Ccris 6119
26. Dtxsid8047898
27. Rebaudioside A [usp-rs]
28. Schembl19769999
29. Sg 95ra50
30. Chebi:145012
31. Ra 95
32. Hy-n0466
33. Rebaudioside A, >=96% (hplc)
34. Mfcd02183463
35. S3796
36. Glycoside A3, From Stevia Rebaudiana
37. Akos037748820
38. Zinc242498440
39. Ccg-270597
40. Cs-5793
41. Db15136
42. As-18742
43. Rebaudioside A, Analytical Reference Material
44. Q63408635
45. Rebaudioside A, United States Pharmacopeia (usp) Reference Standard
46. (4.alpha.)-13-((o-.beta.-d-glucopyranosyl-(1->2)-o-(.beta.-d-glucopyranosyl-(1->3))-.beta.-d-glucopyranosyl)oxy)kaur-16-en-18-oic Acid .beta.-d-glucopyranosyl Ester
47. [(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1r,4s,5r,9s,10r,13s)-13-[(2s,3r,4s,5r,6r)-5-hydroxy-6-(hydroxymethyl)-3,4-bis[[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy]oxan-2-yl]oxy-5,9-dimethyl-14-methylidenetetracyclo[11.2.1.01,10.04,9]hexadecane-5-carboxylate
48. 1-o-(13alpha-{[beta-d-glucopyranosyl-(1->2)-[beta-d-glucopyranosyl-(1->3)]-beta-d-glucopyranosyl]oxy}-18-oxo-5beta,8alpha,9beta,10alpha-kaur-16-en-18-yl)-beta-d-glucopyranose
49. 13-[(2-o-beta-d-glucopyranosyl-3-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl)oxy]-ent-kaur-16-en-19-oic Acid Beta-d-glucopyranosyl Ester
| Molecular Weight | 967.0 g/mol |
|---|---|
| Molecular Formula | C44H70O23 |
| XLogP3 | -2.8 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 23 |
| Rotatable Bond Count | 13 |
| Exact Mass | 966.43078848 g/mol |
| Monoisotopic Mass | 966.43078848 g/mol |
| Topological Polar Surface Area | 374 Ų |
| Heavy Atom Count | 67 |
| Formal Charge | 0 |
| Complexity | 1760 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 26 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
TRADITIONAL MEDICINE: A number of studies have suggested that, beside sweetness, stevioside along with related compounds, which include rebaudioside A (second most abundant component of S. rebaudiana leaf), steviol and isosteviol (metabolic components of stevioside) may also offer therapeutic benefits, as they have anti-hyperglycemic, anti-hypertensive, anti-inflammatory, anti-tumor, anti-diarrheal, diuretic, and immunomodulatory actions. It is of interest to note that their effects on plasma glucose level and blood pressure are only observed when these parameters are higher than normal. As steviol can interact with drug transporters, its role as a drug modulator is proposed...
PMID:19000919 Chatsudthipong V, Muanprasat C; Pharmacol Ther 121 (1): 41-54 (2009)
/EXPL THER/ ...This study was to designed to evaluate the effect of stevioside in human hypertension. A multicentre, randomized, double-blind, placebo-controlled study was undertaken. This study group consisted of 106 Chinese hypertensive subjects with diastolic blood pressure between 95 and 110 mm Hg and ages ranging from 28 to 75 years with 60 subjects (men 34, women 26; mean +/- s.d., 54.1+/-3.8 years) allocated to active treatment and 46 (men 19, women 27; mean +/- s.d., 53.7+/-4.1 years) to placebo treatment. Each subject was given capsules containing stevioside (250 mg) or placebo thrice daily and followed-up at monthly intervals for 1 year. After 3 months, the systolic and diastolic blood pressure of the stevioside group decreased significantly (systolic: /from/166.0+/-9.4 /to/ 152.6+/-6.8 mmHg; diastolic: /from/ 104.7 +/- 5.2 /to/ 90.3+/-3.6 mm Hg, P<0.05), and the effect persisted during the whole year. Blood biochemistry parameters including lipid and glucose showed no significant changes. No significant adverse effect was observed and quality of life assessment showed no deterioration. This study shows that oral stevioside is a well tolerated and effective modality that may be considered as an alternative or supplementary therapy for patients with hypertension. /Stevioside/
PMID:10971305 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2014988 Chan P, et al; Br J Clin Pharmacol 50 (3): 215-20 (2000)
In a study designed to compare the absorption, plasma profiles, metabolism and excretion of (14)C-rebaudioside A, (14)C-stevioside and (14)C-steviol, single oral gavage doses were administered to intact and bile duct-cannulated male and female Sprague-Dawley rats. Doses of 5 mg (14)C-rebaudioside A/kg bw, 4.2 mg (14)C-stevioside/kg bw and 1.6 mg (14)C-steviol/kg bw were administered for the absorption, metabolism and excretion parts of the study; these doses were equal when converted to steviol. In order to determine the plasma profile, three rats per sex per substance were dosed and blood samples taken 0.5, 1, 4, 8, 12 and 24 hr after dosing. Peak plasma concentrations (Cmax) of the three test compounds were recorded at 8, 4 and 0.5 hr following dosing with (14)C-rebaudioside A, (14)C-stevioside and(14)C-steviol, respectively. In the main study, 27 animals per sex per compound were used, and blood samples were taken 0.25, 0.5, 1, 2, 4, 8, 24, 28 and 72 hr after dosing. Concentrations of radioactivity were found to decline between 15 min and 1 hr following dosing with (14)C-rebaudioside A and (14)C-stevioside and then increased from 1 to 2-8 hr before declining again. The Cmax and the area under the plasma concentration-time curve (AUC) of steviol were lower for rebaudioside A than for stevioside, indicating slightly greater formation of steviol from stevioside than from rebaudioside A. Following an oral dose of (14)C-steviol, the Cmax occurred in the first 15 min after administration and declined rapidly between 15 min and 1 hr. A small increase was observed at 2 hr, followed by a further decline. A single dose of test compound was administered to five intact rats per sex and five bile duct-cannulated rats per sex. For intact rats, urine and feces were collected regularly up to 96 hr after dosing. For each cannulated rat, bile, urine and faces were collected regularly up to 48 hr after dosing. Of the total dose in intact rats, 97-98% of (14)C-rebaudioside A and (14)C-stevioside and 90% of (14)C-steviol were recovered in the feces. For all compounds, the majority of the fecal radioactivity was excreted in the first 24 hr after dosing (64-89%), with a further 10-22% excreted in the feces between 24 and 48 hr. No radioactivity was detected in the carcasses of the animals given any of the test compounds at 96 hr after dosing. In cannulated rats, 70-80% of the (14)C-rebaudioside A and (14)C-stevioside dosage was excreted in the bile within 24 hr. The remaining dose was excreted in the feces (21-30%) and in the urine and cage washings (1-2%). The biliary excretion of steviol was more rapid, with 50-70% of the dose eliminated in the first 3 hr after dosing. Only 1-2% of the dose was excreted in the feces, with urine and cage washings accounting for another 1%. /Steviol glycosides/
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 60: Steviol Glycosides p.185-6 (2009). Available from, as of July 21, 2011: https://www.inchem.org/pages/jecfa.html
Five male Sprague-Dawley rats were administered an intravenous injection of 8 mg isosteviol/kg bw. Blood samples were taken immediately prior to dosing and for up to 48 h after dosing. Urine samples were collected up to 24 hr following dosing. Plasma and urine samples were analysed for isosteviol using liquid chromatography/tandem mass spectrometry (LC-MS/MS). Plasma levels declined relatively quickly for 150 min, and then a much slower rate of clearance was observed. Low renal excretion was observed, and a terminal half-life of 406 +/- 31.7 min was calculated. This high terminal half-life was due to a large volume of distribution (suggesting extensive distribution outside of the plasma) and a relatively low rate of clearance. /Isosteviol/
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 60: Steviol Glycosides p.185 (2009). Available from, as of July 21, 2011: https://www.inchem.org/pages/jecfa.html
Five male and five female healthy volunteers (aged 21-29 years) were provided with capsules containing 250 mg stevioside (97% stevioside, 2.8% steviolbioside, 0.2% rebaudioside A) to be taken 3 times per day for 3 days. Doses, expressed as steviol, were 299 mg/day or 4.60 mg/kg bw per day for females and 4.04 mg/kg bw per day for males. Twenty-four-hour urine samples were taken at enrollment and after dosing. Urine samples were analyzed for bound steviol and steviol glucuronide. Blood samples were also taken before and after dosing and analyzed for alkaline phosphatase, alanine aminotransferase (ALT), glutamic- pyruvic transaminase (GPT), creatine kinase and lactate dehydrogenase. No significant differences in electrolytes or markers of tissue damage were observed. The only metabolite detected in urine was steviol glucuronide. /It was/ concluded that because of its molecular size, the uptake of stevioside by the intestinal tract is likely to be very low and that stevioside is not degraded by enzymes in the gastrointestinal tract. However, bacteria found in the gut microflora are able to metabolize stevioside into free steviol, which is easily absorbed. /It is/ suggested that following degradation by the microflora, part of the steviol is absorbed by the colon and transported to the liver by portal blood, where it is conjugated with glucuronide, which is subsequently excreted in the urine. /Stevioside/
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 60: Steviol Glycosides p.187 (2009). Available from, as of July 21, 2011: https://www.inchem.org/pages/jecfa.html
(14)C-rebaudioside A, (14)C-stevioside and (14)C-steviol were administered by gavage to intact and bile duct- cannulated male and female Sprague-Dawley rats, the fecal metabolite profiles were similar between the three test substances, with the predominant metabolite being steviol in all cases, with a smaller amount of steviol glucuronide being found, along with a very small percentage of unidentifiable metabolites. Steviol glucuronide was the predominant radioactive component in the bile, indicating that deconjugation occurs in the lower intestine. /Steviol glycosides/
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 60: Steviol Glycosides p.187 (2009). Available from, as of July 21, 2011: https://www.inchem.org/pages/jecfa.html
The metabolism of stevioside (purity not stated) was investigated in human saliva, gastric secretions and fecal bacteria, as well as intestinal brush border membranes and intestinal microflora from rats, mice and hamsters. Stevioside was unchanged following incubation with human saliva and gastric secretions or with intestinal brush border membrane vesicles from rats, mice and hamsters. Microflora from rats, mice, hamsters and humans was found to metabolize stevioside to steviol. Steviol-16,17alpha-epoxide was found to be produced by human fecal bacteria, but this was converted back to steviol by further action of fecal bacteria. /Stevioside/
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 60: Steviol Glycosides p.187 (2009). Available from, as of July 21, 2011: https://www.inchem.org/pages/jecfa.html
Five male Sprague-Dawley rats were administered an intravenous injection of 8 mg isosteviol/kg bw. Blood samples were taken immediately prior to dosing and for up to 48 hr after dosing. ... A terminal half-life of 406 +/- 31.7 min was calculated. This high terminal half-life was due to a large volume of distribution (suggesting extensive distribution outside of the plasma) and a relatively low rate of clearance. /Isosteviol/
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 60: Steviol Glycosides p.185 (2009). Available from, as of July 21, 2011: https://www.inchem.org/pages/jecfa.html


