
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
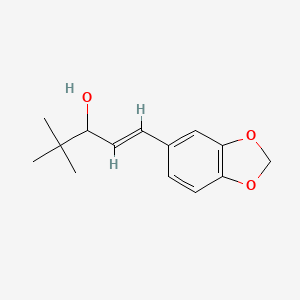

1. D-306
2. Diacomit
1. 49763-96-4
2. Diacomit
3. Bcx 2600
4. 1-(1,3-benzodioxol-5-yl)-4,4-dimethyl-1-penten-3-ol
5. 137767-55-6
6. Bcx-2600
7. 1-(benzo[d][1,3]dioxol-5-yl)-4,4-dimethylpent-1-en-3-ol
8. 4,4-dimethyl-1-((3,4-methylenedioxy)phenyl)-1-penten-3-ol
9. (1e)-1-(2h-1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol
10. Ncgc00185769-01
11. Estiripentol
12. 1-penten-3-ol, 4,4-dimethyl-1-(3,4-methylenedioxyphenyl)-
13. (e)-1-(benzo[d][1,3]dioxol-5-yl)-4,4-dimethylpent-1-en-3-ol
14. 1-penten-3-ol, 1-(1,3-benzodioxol-5-yl)-4,4-dimethyl-
15. Stiripentolum
16. Stiripentol [mi]
17. Stiripentol [inn]
18. Stiripentol [jan]
19. Stiripentol [usan:inn]
20. Stiripentol [usan]
21. Stiripentolum [inn-latin]
22. Estiripentol [inn-spanish]
23. Stiripentol [mart.]
24. 4,4-dimethyl-1-(3,4-methylenedioxyphenyl)-1-penten-3-ol
25. Smr000449279
26. Stiripentol [who-dd]
27. Stiripentol [ema Epar]
28. R02xot8v8i
29. Einecs 256-480-9
30. Mfcd00869310
31. Brn 1313047
32. (e)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol
33. Diacomit (tn)
34. Me-2080
35. Cpd000449279
36. Dsstox_cid_28994
37. Dsstox_rid_83259
38. Dsstox_gsid_49068
39. 5-19-02-00640 (beilstein Handbook Reference)
40. Mls000758313
41. Mls001424144
42. Schembl216436
43. Stiripentol (jan/usan/inn)
44. Gtpl5469
45. Schembl2533815
46. Chembl1983350
47. Chebi:94435
48. Stiripentol, >=98% (hplc)
49. Dtxsid80860609
50. Stiripentol [orange Book]
51. Hms2052k07
52. Hms2232p06
53. Hms3886m17
54. Bcp10434
55. Tox21_113622
56. Bdbm50504273
57. S5266
58. Akos025149123
59. Akos027255159
60. Ccg-101092
61. Ccg-266819
62. Cs-7801
63. Db09118
64. Nc00342
65. Ncgc00185769-02
66. Bs-16863
67. Cas-49763-96-4
68. Hy-103392
69. D05928
70. W10731
71. Bcx2600; Bcx-2600; Bcx 2600
72. 763s964
73. Q412182
74. (1e)-1-(1,3-benzodioxol-5-yl)-4,4-dimethyl-1-penten-3-ol
75. (e)-1-(3,4-methylenedioxyphenyl)-4,4-dimethyl-1-penten-3-ol
76. 131206-47-8
| Molecular Weight | 234.29 g/mol |
|---|---|
| Molecular Formula | C14H18O3 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 234.125594432 g/mol |
| Monoisotopic Mass | 234.125594432 g/mol |
| Topological Polar Surface Area | 38.7 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 280 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for use in conjunction with clobazam and valproate as adjunctive therapy of refractory generalized tonic-clonic seizures in patients with severe myoclonic epilepsy in infancy (SMEI, Dravets syndrome) whose seizures are not adequately controlled with clobazam and valproate.
Diacomit is indicated for use in conjunction with clobazam and valproate as adjunctive therapy of refractory generalized tonic-clonic seizures in patients with severe myoclonic epilepsy in infancy (SMEI, Dravet's syndrome) whose seizures are not adequately controlled with clobazam and valproate.
Stiripentol is an orphan drug that effectively reduces seizure frequency in infantile epilepsy as an adjunct therapy and also exhibits a therapeutic advantage in improving the efficacy of other antiepileptic drugs. It potentiates GABA transmission by elevating the levels of the inhibitory neurotransmitters in the brain. Stiripentol is a positive allosteric modulator of GABA-A receptors in the brain that enhances the opening duration of the channel by binding to a site different than the benzodiazepine binding site. Reduced synaptosomal uptake of GABA and/or inhibition of GABA transaminase may also explain the role of stiripentol in reducing the events of seizure. The anticonvulsant activity of stiripentol is age-dependent, with increased efficacy in younger patients.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AX17
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX17 - Stiripentol
Absorption
Absorption of stiripentol is quick with the peak plasma concentration reached within 1.5 hours following oral administration. The systemic exposure increases in a dose-proportional relationship. It is rapidly taken up into the brain and enters the cerebellum and medulla. It displays low bioavailability due to water insolubility and metabolism.
Route of Elimination
Renal elimination is mainly responsible for excretion of stiripentol. About 73% of total administered dose is found in urine as metabolites, while further 13-24% of the total dose is recovered in faeces as unchanged substance.
Volume of Distribution
The average volume of distribution is 1.03 L/kg but does not display a dose-dependent relationship. It is expected to be distributed into the extravascular space and with a high degree of tissue binding.
Clearance
Plasma clearance decreases markedly at high doses; it falls from approximately 40 L/kg/day at the dose of 600 mg/day to about 8 L/kg/day at the dose of 2,400 mg. Decreased clearance is probably explained by inhibition of the cytochrome P450 isoenzymes that catalyzes stiripentol metabolism.
There are 13 metabolites from extensive metabolism stiripentol that are found in urine. The predominant metabolic pathways involve demethylenation and glucuronidation. Other metabolic pathways are oxidative cleavage of the methylenedioxy ring system, O-methylation of catechol metabolites, hydroxylation of the t-butyl group and conversion of the allylic alcohol side-chain to the isomeric 3-pentanone structure. Based on in vitro studies, phase I metabolism of stiripentol involves enzymatic activity of CYP1A2, CYP2C19 and CYP3A4.
Elimination half life is approximately ranges from 4.5 to 13 hours, in a dose-dependent manner.
Stiripentol enhances GABAergic inhibition and prolongs the open duration of GABA-A receptor chloride channels by a barbiturate-like mechanism. It binds to GABA-A receptors containing any of the , , , or -subunits but displays strongest potency when bound to receptors containing 3 or subunits. Stiripentol is an inhibitor of lactate dehydrogenase (LDH), which plays a physiological role in energy metabolism of neurons and regulation of neuronal excitation. It binds to the site separate from lactate and pyruvate binding sites of the enzyme and inhibits both pyruvate-to-lactate conversion and lactate-to-pyruvate conversion. LDH inhibitors including stiripentol as antiepileptic treatments mimic ketogenic diet, where the energy source in the brain is switched from glucose to mainly ketone bodies. The ketone bodies directly regulate neuronal excitation and seizures via ATP-sensitive potassium channels and vesicular glutamate transporters. As a potent inhibitor of hepatic cytochrome P450 enzymes, mainly CYP3A4 and CYP2C19, stiripentol co-administration with other antiepileptic drugs elevates the free unchanged active drugs (such as carbamazepine, sodium valproate, phenytoin, phenobarbital and many benzodiazepines) in the circulation to mediate their therapeutic actions.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
72
PharmaCompass offers a list of Stiripentol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Stiripentol manufacturer or Stiripentol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Stiripentol manufacturer or Stiripentol supplier.
PharmaCompass also assists you with knowing the Stiripentol API Price utilized in the formulation of products. Stiripentol API Price is not always fixed or binding as the Stiripentol Price is obtained through a variety of data sources. The Stiripentol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Stiripentol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Stiripentol, including repackagers and relabelers. The FDA regulates Stiripentol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Stiripentol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Stiripentol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Stiripentol supplier is an individual or a company that provides Stiripentol active pharmaceutical ingredient (API) or Stiripentol finished formulations upon request. The Stiripentol suppliers may include Stiripentol API manufacturers, exporters, distributors and traders.
click here to find a list of Stiripentol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Stiripentol DMF (Drug Master File) is a document detailing the whole manufacturing process of Stiripentol active pharmaceutical ingredient (API) in detail. Different forms of Stiripentol DMFs exist exist since differing nations have different regulations, such as Stiripentol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Stiripentol DMF submitted to regulatory agencies in the US is known as a USDMF. Stiripentol USDMF includes data on Stiripentol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Stiripentol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Stiripentol suppliers with USDMF on PharmaCompass.
A Stiripentol written confirmation (Stiripentol WC) is an official document issued by a regulatory agency to a Stiripentol manufacturer, verifying that the manufacturing facility of a Stiripentol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Stiripentol APIs or Stiripentol finished pharmaceutical products to another nation, regulatory agencies frequently require a Stiripentol WC (written confirmation) as part of the regulatory process.
click here to find a list of Stiripentol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Stiripentol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Stiripentol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Stiripentol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Stiripentol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Stiripentol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Stiripentol suppliers with NDC on PharmaCompass.
Stiripentol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Stiripentol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Stiripentol GMP manufacturer or Stiripentol GMP API supplier for your needs.
A Stiripentol CoA (Certificate of Analysis) is a formal document that attests to Stiripentol's compliance with Stiripentol specifications and serves as a tool for batch-level quality control.
Stiripentol CoA mostly includes findings from lab analyses of a specific batch. For each Stiripentol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Stiripentol may be tested according to a variety of international standards, such as European Pharmacopoeia (Stiripentol EP), Stiripentol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Stiripentol USP).
