
Synopsis
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
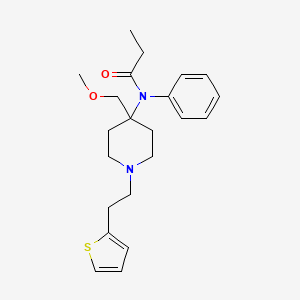

1. Citrate, Sufentanil
2. Curasan, Sufentanil
3. R 30730
4. R-30730
5. R30730
6. Sufenta
7. Sufentanil Citrate
8. Sufentanil Curasan
9. Sufentanil Hameln
10. Sufentanil Ratiopharm
11. Sufentanil-hameln
12. Sufentanil-ratiopharm
13. Sufentanilhameln
14. Sufentanilratiopharm
15. Sulfentanil
16. Sulfentanyl
1. Sufentanyl
2. 56030-54-7
3. Sufentanilum
4. Sufentanilum [inn-latin]
5. Zalviso
6. R-30730
7. N-(4-(methoxymethyl)-1-(2-(2-thienyl)ethyl)-4-piperidyl)propionanilide
8. N-(4-(methoxymethyl)-1-(2-(2-thienyl)ethyl)-4-piperidinyl)-n-phenylpropanamide
9. Chebi:9316
10. Afe2yw0iiz
11. N-[4-(methoxymethyl)-1-(2-thiophen-2-ylethyl)piperidin-4-yl]-n-phenylpropanamide
12. Chronogesic
13. Ids-ns-001
14. R 30,730
15. Propanamide, N-(4-(methoxymethyl)-1-(2-(2-thienyl)ethyl)-4-piperidinyl)-n-phenyl-
16. Propanamide, N-[4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidinyl]-n-phenyl-
17. N-[4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidyl]propionanilide
18. R 30730
19. Hsdb 6760
20. Unii-afe2yw0iiz
21. Sufentanil (usan/inn)
22. Sufentanil [usan:inn:ban]
23. Sufentanil Base
24. N-[4-(methoxymethyl)-1-{2-(2-thienyl)ethyl}-4-piperidinyl]-n-phenylpropanamide
25. Dsuvia (brand Name)
26. Sufenta (brand Name)
27. Sufentanil [mi]
28. Sufentanil [inn]
29. Sufentanil [hsdb]
30. Sufentanil [usan]
31. Sufentanil [vandf]
32. Chembl658
33. Epitope Id:153513
34. Sufentanil [mart.]
35. Arx04
36. Sufentanil [who-dd]
37. Oprea1_120838
38. Oprea1_246787
39. Schembl26728
40. Cid_65494
41. Arx 04
42. Gtpl3534
43. Dtxsid6023604
44. Bdbm94503
45. Sufentanil [ep Monograph]
46. Zinc538386
47. Pdsp1_001741
48. Pdsp2_001724
49. Db00708
50. N-{4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]piperidin-4-yl}-n-phenylpropanamide
51. N-{4-[(methyloxy)methyl]-1-[2-(2-thienyl)ethyl]piperidin-4-yl}-n-phenylpropanamide
52. Ncgc00247355-01
53. Hy-13754
54. C08022
55. D05938
56. L000580
57. Q417915
58. N-[4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidinyl]propionalnalide
59. Citric Acid;n-[4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidyl]-n-phenyl-propionamide
60. N-(4-(methoxymethyl)-1-(2-(thiophen-2-yl)ethyl)piperidin-4-yl)-n-phenylpropionamide
61. N-(4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidinyl)-n-phenylpropanamide #
62. N-[4-(methoxymethyl)-1-[2-(thiophen-2-yl)ethyl]piperidin-4-yl]-n-phenylpropanamide
63. 2-hydroxypropane-1,2,3-tricarboxylic Acid;n-[4-(methoxymethyl)-1-(2-thiophen-2-ylethyl)-4-piperidinyl]-n-phenylpropanamide
64. N-[4-(methoxymethyl)-1-(2-thiophen-2-ylethyl)piperidin-4-yl]-n-phenyl-propanamide;2-oxidanylpropane-1,2,3-tricarboxylic Acid
| Molecular Weight | 386.6 g/mol |
|---|---|
| Molecular Formula | C22H30N2O2S |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 386.20279938 g/mol |
| Monoisotopic Mass | 386.20279938 g/mol |
| Topological Polar Surface Area | 61 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 459 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics, Opioid; Adjuvants, Anesthesia; Narcotics; Anesthetics, Intravenous
National Library of Medicine's Medical Subject Headings. Sufentanil. Online file (MeSH, 2017). Available from, as of April 26, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Sufentanil is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of June 20, 2017: https://clinicaltrials.gov/
Sufentanil Citrate Injection is indicated for intravenous administration in adults and pediatric patients as an analgesic adjunct in the maintenance of balanced general anesthesia in patients who are intubated and ventilated. /Included in US product label/
NIH; DailyMed. Current Medication Information for Sufentanil Citrate Injection (Updated: December 2016). Available from, as of May 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95e639db-1ca3-4328-b347-7ea348f6cc72
Sufentanil Citrate Injection is indicated for intravenous administration in adults and pediatric patients ... as a primary anesthetic agent for the induction and maintenance of anesthesia with 100% oxygen in patients undergoing major surgical procedures, in patients who are intubated and ventilated, such as cardiovascular surgery or neurosurgical procedures in the sitting position, to provide favorable myocardial and cerebral oxygen balance or when extended postoperative ventilation is anticipated. /Included in US product label/
NIH; DailyMed. Current Medication Information for Sufentanil Citrate Injection (Updated: December 2016). Available from, as of May 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95e639db-1ca3-4328-b347-7ea348f6cc72
For more Therapeutic Uses (Complete) data for Sufentanil (7 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: ADDICTION, ABUSE, AND MISUSE. Sufentanil citrate injection exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient's risk before prescribing and monitor regularly for these behaviors and conditions.
NIH; DailyMed. Current Medication Information for Sufentanil Citrate Injection (Updated: December 2016). Available from, as of May 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95e639db-1ca3-4328-b347-7ea348f6cc72
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Sufentanil Citrate Injection should be administered only by persons specifically trained in the use of anesthetic drugs and the management of the respiratory effects of potent opioids, including respiration and cardiac resuscitation of patients in the age group being treated. Such training must include the establishment and maintenance of a patent airway and assisted ventilation. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered anesthetic doses of Sufentanil Citrate Injection. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
NIH; DailyMed. Current Medication Information for Sufentanil Citrate Injection (Updated: December 2016). Available from, as of May 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95e639db-1ca3-4328-b347-7ea348f6cc72
Sufentanil should be used with caution in patients with head injuries, since the drug may obscure the clinical course in these patients. The drug should be used with caution in patients with pulmonary disease, decreased respiratory reserve, or potentially compromised respiratory function, since opiate agonists may cause additional decreases in respiratory function and increases in airway resistance in these patients. These adverse respiratory effects can be managed by using assisted or controlled respiration during anesthesia. Sufentanil-induced respiratory depression can be reversed by administration of an opiate antagonist (e.g., naloxone); however, the duration of respiratory depression produced by sufentanil may be longer than the duration of the opiate antagonist and, therefore, appropriate patient monitoring should be continued following apparent initial reversal.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2279
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Sufentanil Citrate Injection and any potential adverse effects on the breastfed infant from Sufentanil Citrate Injection or from the underlying maternal condition.
NIH; DailyMed. Current Medication Information for Sufentanil Citrate Injection (Updated: December 2016). Available from, as of May 2, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95e639db-1ca3-4328-b347-7ea348f6cc72
For more Drug Warnings (Complete) data for Sufentanil (22 total), please visit the HSDB record page.
The indications for this drug are as follows: 1. As an analgesic adjunct in the maintenance of balanced general anesthesia in patients who are intubated and ventilated. 2. As a primary anesthetic agent for the induction and maintenance of anesthesia with 100% oxygen in patients undergoing major surgical procedures, in patients who are intubated and ventilated, such as cardiovascular surgery or neurosurgical procedures in the sitting position, to provide favorable myocardial and cerebral oxygen balance or when extended postoperative ventilation is anticipated. 3. For epidural administration as an analgesic combined with low dose (usually 12.5 mg per administration) bupivacaine usually during labor and vaginal delivery 4. The sublingual form is indicated for the management of acute pain in adults that is severe to warrant the use of an opioid analgesic in certified medically supervised healthcare settings, including hospitals, surgical centers, and emergency departments.
FDA Label
Zalviso is indicated for the management of acute moderate to severe post-operative pain in adult patients.
**Effect on the Central Nervous System (CNS)** In clinical settings, sufentanil exerts its principal pharmacologic effects on the central nervous system. Its primary therapeutic actions are analgesia and sedation. Sufentanil may increase pain tolerance and decrease the perception of pain. This drug depresses the respiratory centers, depresses the cough reflex, and constricts the pupils,. When used in balanced general anesthesia, sufentanil has been reported to be as much as 10 times as potent as fentanyl. When administered intravenously as a primary anesthetic agent with 100% oxygen, sufentanil is approximately 5 to 7 times as potent as fentanyl. High doses of intravenous sufentanil have been shown to cause muscle rigidity, likely as a result of an effect on the substantia nigra and the striate nucleus in the brain. Sleep-inducing (hypnotic) activity can be demonstrated by EEG alterations. **Effects on the Respiratory System** Sufentanil may cause respiratory depression. **Effects on the Cardiovascular System** Sufentanil causes peripheral vasodilation which may result in orthostatic hypotension or syncope. Bradycardia may also occur. Clinical signs or symptoms of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes and sweating and/or orthostatic hypotension. **Effects on the Gastrointestinal Tract** Sufentanil causes a reduction in motility associated with an increase in smooth muscle tone in both the antrum of the stomach and duodenum. Digestion of food in the small intestine may be delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased and lead to spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of the sphincter of Oddi, as well as temporary elevations in serum amylase.
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
N01AH03
N01AH03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AH - Opioid anesthetics
N01AH03 - Sufentanil
Absorption
Bioavailability of a single sublingual tablet was 52%, decreasing to 35% with repeat dosing. After epidural administration of incremental doses totaling 5 to 40 mcg sufentanil during labor and delivery, maternal and neonatal sufentanil plasma concentrations were at or near the 0.05 to 0.1 ng/mL limit of detection, and were slightly higher in mothers than in their infants.
Route of Elimination
Approximately 80% of the administered dose is excreted within 24 hours and only 2% of the dose is eliminated as unchanged drug.
Volume of Distribution
Sufentanil has a distribution time of 1.4 minutes and redistribution time of 17.1 minutes. The central volume of distribution after intravenous application of sufentanil is approximately 14 L and the volume of distribution at steady state is approximately 350 L.
Clearance
The total plasma clearance after single intravenous administration is about 917 l/min. The clearance of sufentanil in healthy neonates is approximately one-half that in adults and children. The clearance rate of sufentanil can be further reduced by up to a third in neonates with cardiovascular disease.
This randomized double-blind investigation was designed to study the placental transfer and neonatal effects of epidural sufentanil and fentanyl infused with bupivacaine for labor analgesia. Healthy parturient women (n = 36) received epidural bupivacaine alone (group B) or with fentanyl (group B-F) or sufentanil (group B-S). Group B received a 12-ml bolus of 0.25% bupivacaine followed by a 10 mL/hr infusion of 0.125% bupivacaine. Groups B-F and B-S received a 12-mL bolus of 0.125% bupivacaine with 75 ug fentanyl or 15 ug sufentanil, respectively, followed by 10 mL/hr of 0.125% bupivacaine with fentanyl 1.5 ug/mL or sufentanil 0.25 ug/mL. Maternal venous (MV) and umbilical arterial (UA) and umbilical venous (UV) bupivacaine and opioid plasma concentrations were determined. Neonatal assessment included Apgar scores, umbilical cord blood gas analyses, and neurobehavioral testing at delivery and at 2 and 24 hr of life using the Neurologic and Adaptive Capacity Score (NACS). The mean total dose of fentanyl was 136.6 +/- 13.1 micrograms (SEM), and of sufentanil, 23.8 +/- 1.8 micrograms. Although administered in a ratio of 5.7:1, fentanyl and sufentanil MV plasma concentrations were in the ratio of 27:1. UV/MV ratios were 0.37 for fentanyl and 0.81 for sufentanil. Fentanyl was detected in most UA samples, whereas sufentanil was present in only one sample. Neonatal condition was good and generally similar in all groups, with the exception of a lower NACS at 24 h in group B-F. Although the degree of placental transfer of sufentanil appeared greater than that of fentanyl, lower MV sufentanil concentrations resulted in less fetal exposure to sufentanil. The lower NACS at 24 hr in group B-F may reflect the continued presence of fentanyl in the neonate.
PMID:7631952 Loftus JR et al; Anesthesiology 83 (2): 300-8 (1995)
The effects of cirrhosis on the elimination kinetics and plasma protein binding of sufentanil were evaluated in 12 anesthetized patients with uncomplicated cirrhosis and these findings were compared with data from age-matched control anesthetized patients with normal hepatic and renal function. Sufentanil 3 ug/kg was given iv as a bolus injection and venous plasma concentrations were measured at intervals up to 10 hr. The average (+ or - standard deviation) elimination half life was 3.5 + or - 0.9 hr in controls and did not differ in cirrhotics: 4.1 + or - 0.6 hr. The plasma clearance did not differ between the two groups: 11.3 + or - 2.5 ml/min kg in controls and 10.8 + or - 4.6 ml/min kg in cirrhotic patients. The sufentanil free fraction was also similar in controls (8.3 + or - 1.5%) and in cirrhotic patients (9.6 + or - 1.8%). These data suggest that sufentanil in a single dose should have a similar duration of action in patients with uncomplicated cirrhosis and in normal patients.
PMID:2521279 Chauvin M et al; Anesth Analg 68 (1): 1-4 (1989)
The in vitro plasma protein binding and distribution in blood of fentanyl and three analogues were studied in rats, dogs and healthy volunteers. In human plasma, 84.4% of fentanyl was bound, 92.5% of sufentanil, 92.1% of alfentanil and 93.6% of lofentanil. Plasma protein binding of the four analgesics was independent of their concentration over the whole therapeutic range. Plasma protein binding of alfentanil was much less pH dependent than that of the three other analgesics. Attention was drawn to the possible contribution of the "acute phase' protein alpha 1-acid glycoprotein (alpha 1-AGP), of lipoproteins and of blood cells to the binding of fentanyl and its analogues in blood.
PMID:6214227 Meuldermans WE et al; Arch Int Pharmacodyn Ther. 1982 May;257(1):4-19 (1982)
The aim of this study was to compare the pharmacokinetics of fentanyl, alfentanil, and sufentanil in isoflurane-anesthetized cats. Six adult cats were used. Anesthesia was induced and maintained with isoflurane in oxygen. End-tidal isoflurane concentration was set at 2% and adjusted as required due to spontaneous movement. Fentanyl (10 ug/kg), alfentanil (100 ug/kg), or sufentanil (1 ug/kg) was administered intravenously as a bolus, on separate days. Blood samples were collected immediately before and for 8 hr following drug administration. Plasma drug concentration was determined using liquid chromatography/mass spectrometry. Compartment models were fitted to concentration-time data. A 3-compartment model best fitted the concentration-time data for all drugs, except for 1 cat in the sufentanil group (excluded from analysis). The volume of the central compartment and the volume of distribution at steady-state (L/kg) [mean +/- SEM (range)], the clearance (mL/min/kg) [harmonic mean +/- pseudo-SD (range)], and the terminal half-life (min) [median (range)] were 0.25 +/- 0.04 (0.09-0.34), 2.18 +/- 0.16 (1.79-2.83), 18.6 +/- 5.0 (15-29.8), and 151 (115-211) for fentanyl; 0.10 +/- 0.01 (0.07-0.14), 0.89 +/- 0.16 (0.68-1.83), 11.6 +/- 2.6 (9.2-15.8), and 144 (118-501) for alfentanil; and 0.06 +/- 0.01 (0.04-0.10), 0.77 +/- 0.07 (0.63-0.99), 17.6 +/- 4.3 (13.9-24.3), and 54 (46-76) for sufentanil. Differences in clearance and volume of distribution result in similar terminal half-lives for fentanyl and alfentanil, longer than for sufentanil.
PMID:23895731 Pypendop BH et al; J Vet Pharmacol Ther 37 (1): 13-7 (2014)
For more Absorption, Distribution and Excretion (Complete) data for Sufentanil (18 total), please visit the HSDB record page.
The liver and small intestine are the major sites of biotransformation. Sufentanil is rapidly metabolized to a number of inactive metabolites, with oxidative N- and O-dealkylation being the major routes of elimination.
Although the metabolic fate of sufentanil in humans has not been fully characterized, the drug appears to be metabolized mainly in the liver and small intestine via N-dealkylation at the piperidine nitrogen and O-demethylation. The O-demethylated metabolite appears to have about 10% of the analgesic activity of the unchanged drug. The hepatic extraction ratio of the drug (EH) has been reported to be about 0.72 following IV administration of a single 5-mcg/kg dose.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2281
The elimination half-life is 164 minutes in adults when administered intravenously (IV). The elimination half-life of sufentanil is shorter (e.g. 97 +/- 42 minutes) in infants and children, and longer in neonates (e.g. 434 +/- 160 minutes) compared to that of adolescents and adults. After a single administration of a 15 microgram sufentanil sublingual tablet, mean terminal phase half-lives in the range of 6-10 hours have been observed. After multiple administrations, a longer average terminal half-life of up to 18 hours was measured, owing to the higher plasma concentrations of sufentanil achieved after repeated dosing and due to the possibility to quantify these concentrations over a longer time period.
In adults with normal renal and hepatic function, the plasma half-life in the initial (distribution) phase averages 0.72-1.2 minutes, the plasma half-life in the second (redistribution) phase averages 13.7-17 minutes, and the plasma half-life in the terminal (elimination) phase averages 140-158 minutes. The elimination half-life is longer (434 minutes) in neonates but shorter in infants and children (97 minutes), compared with adults and adolescents.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2281
Sufentanil is a synthetic, potent opioid with highly selective binding to -opioid receptors. These receptors are widely distributed in the human brain, spinal cord, and other tissues,. In general, opioids decrease cAMP (affecting neural signaling pathways), decrease neurotransmitter release, and cause membrane hyperpolarization, all of which contribute to the relief of painful symptoms. Opiate receptors are coupled with G-protein receptors and function as both positive and negative regulators of synaptic neural transmission via G-proteins that activate effector proteins. Binding of the opiate receptor leads to the exchange of GTP for GDP on the G-protein complex. As the effector system is adenylate cyclase and cAMP, located at the inner surface of the plasma membrane, opioids decrease intracellular cAMP by inhibiting adenylate cyclase. The release of nociceptive neurotransmitters such as substance P, GABA, dopamine, acetylcholine, and noradrenaline is then inhibited. Opioids close N-type voltage-operated calcium channels (OP2-receptor agonist), also preventing neurotransmitter release. Sufentanil and other opioids open calcium-dependent inwardly rectifying potassium channels, resulting in hyperpolarization and reduced neuronal excitability,.
We determined the binding domains of sufentanil and lofentanil in the mu opioid receptor by comparing their binding affinities to seven mu/delta and six mu/kappa chimeric receptors with those to mu, delta and kappa opioid receptors. TMHs 6 and 7 and the e3 loop of the mu opioid receptor were important for selective binding of sufentanil and lofentanil to the mu over the kappa receptor. TMHs 1-3 and the e1 loop of the mu opioid receptor conferred binding selectivity for sufentanil over the delta receptor. Thus, the region that conferred binding selectivity for sufentanil differs, depending on chimeras used. In addition, the interaction TMHs 1-3 and TMHs 6-7 was crucial for the high affinity binding of these two ligands. These two regions are likely to contain sites of interaction with the ligands or to confer conformations specific to the mu receptor.
PMID:8612823 Zhu J et al; FEBS Lett 384 (2): 198-202 (1996)
Opioids are the most effective and widely used drugs in the treatment of severe acute and chronic pain. They act through opioid receptors that belong to the family of G protein-coupled receptors. Three classes of opioid receptors (mu, delta, kappa), expressed in the central and peripheral nervous system, have been identified. The analgesic effect of opioids is mediated through multiple pathways of opioid receptor signaling (e.g., G(i/o) coupling, cAMP inhibition, Ca(++) channel inhibition). The standard exogenous opioid analgesics used in the operating room are fentanyl, sufentanil, morphine, alfentanil, and remifentanil.
PMID:18685878 Zollner C, Schafer M; Anaesthesist 57 (7): 729-40 (2008)
Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
60
PharmaCompass offers a list of Sufentanil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sufentanil manufacturer or Sufentanil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sufentanil manufacturer or Sufentanil supplier.
PharmaCompass also assists you with knowing the Sufentanil API Price utilized in the formulation of products. Sufentanil API Price is not always fixed or binding as the Sufentanil Price is obtained through a variety of data sources. The Sufentanil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sufentanil Citrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sufentanil Citrate, including repackagers and relabelers. The FDA regulates Sufentanil Citrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sufentanil Citrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sufentanil Citrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sufentanil Citrate supplier is an individual or a company that provides Sufentanil Citrate active pharmaceutical ingredient (API) or Sufentanil Citrate finished formulations upon request. The Sufentanil Citrate suppliers may include Sufentanil Citrate API manufacturers, exporters, distributors and traders.
click here to find a list of Sufentanil Citrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sufentanil Citrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Sufentanil Citrate active pharmaceutical ingredient (API) in detail. Different forms of Sufentanil Citrate DMFs exist exist since differing nations have different regulations, such as Sufentanil Citrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sufentanil Citrate DMF submitted to regulatory agencies in the US is known as a USDMF. Sufentanil Citrate USDMF includes data on Sufentanil Citrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sufentanil Citrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sufentanil Citrate suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Sufentanil Citrate Drug Master File in Korea (Sufentanil Citrate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Sufentanil Citrate. The MFDS reviews the Sufentanil Citrate KDMF as part of the drug registration process and uses the information provided in the Sufentanil Citrate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Sufentanil Citrate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Sufentanil Citrate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Sufentanil Citrate suppliers with KDMF on PharmaCompass.
A Sufentanil Citrate CEP of the European Pharmacopoeia monograph is often referred to as a Sufentanil Citrate Certificate of Suitability (COS). The purpose of a Sufentanil Citrate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Sufentanil Citrate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Sufentanil Citrate to their clients by showing that a Sufentanil Citrate CEP has been issued for it. The manufacturer submits a Sufentanil Citrate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Sufentanil Citrate CEP holder for the record. Additionally, the data presented in the Sufentanil Citrate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Sufentanil Citrate DMF.
A Sufentanil Citrate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Sufentanil Citrate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Sufentanil Citrate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sufentanil Citrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sufentanil Citrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sufentanil Citrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sufentanil Citrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sufentanil Citrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sufentanil Citrate suppliers with NDC on PharmaCompass.
Sufentanil Citrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sufentanil Citrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sufentanil Citrate GMP manufacturer or Sufentanil Citrate GMP API supplier for your needs.
A Sufentanil Citrate CoA (Certificate of Analysis) is a formal document that attests to Sufentanil Citrate's compliance with Sufentanil Citrate specifications and serves as a tool for batch-level quality control.
Sufentanil Citrate CoA mostly includes findings from lab analyses of a specific batch. For each Sufentanil Citrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sufentanil Citrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Sufentanil Citrate EP), Sufentanil Citrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sufentanil Citrate USP).
