
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
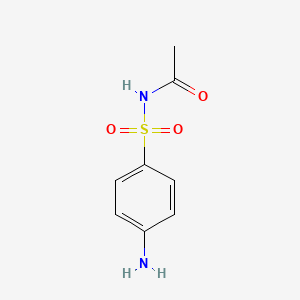

1. Acetopt
2. Acetylsulfanilamide
3. Ak Sulf
4. Ak-sulf
5. Albucid
6. Antbor
7. Belph 10
8. Belph-10
9. Bleph
10. Ceta Sulfa
11. Cetamide
12. Colircusi Sulfacetamida
13. Coliriocilina Sulfacetam
14. Isopto Cetamide
15. Sodium Sulamyd
16. Sulamyd, Sodium
17. Sulf 10
18. Sulf-10
19. Sulfacetam, Coliriocilina
20. Sulfacetamida, Colircusi
21. Sulfacetamide Monosodium Salt
22. Sulfacetamide Sodium
23. Sulfacetamide, Monosodium Salt, Anhydrous
24. Sulfacil
25. Sulfacyl
26. Sulfair
27. Sulphacetamide
1. 144-80-9
2. Sulphacetamide
3. Acetosulfamine
4. N-((4-aminophenyl)sulfonyl)acetamide
5. N-acetylsulfanilamide
6. N-sulfanilylacetamide
7. Albucid
8. Acetosulfamin
9. Urosulfon
10. N-sulphanilylacetamide
11. Sulfacetimide
12. Acetocid
13. Sulfacet
14. Sulfacyl
15. Sulfanilacetamide
16. Formosulfacetamide
17. N-(4-aminophenyl)sulfonylacetamide
18. Albamine
19. Sulphasil
20. Urosulfone
21. Alesten
22. Oclucid
23. Region
24. Sulamyd
25. Sulfanilazetamid
26. P-aminobenzenesulfonacetamide
27. Sulf-10
28. Bleph-10
29. N-[(4-aminophenyl)sulfonyl]acetamide
30. N-acetyl-4-aminobenzenesulfonamide
31. Sulfacetamidum
32. Sulphacetamidum
33. Steramide
34. Sebizon
35. Isopto Cetamide
36. Ophthel-s
37. P-aminobenzenesulfonoacetamide
38. N'-acetylsulfanilamide
39. Sulfacetamida
40. Bleph-10 Liquifilm
41. Op-sulfa 30
42. Sulfacylum
43. Sulfacel-15
44. Acetamide, N-sulfanilyl-
45. Klaron
46. N-((p-aminophenyl)sulfonyl)acetamide
47. N(sup1)-acetylsulfanilamide
48. A-500
49. Ocusulf-10
50. Sulfair-15
51. Sulten-10
52. N-(4-aminophenylsulfonyl)acetamide
53. Sulster
54. Sulf-15
55. N-[(p-aminophenyl)sulfonyl]acetamide
56. Fml-s
57. N(sup 1)-acetyl-4-aminophenylsulfonamide
58. Sulfanilamide, N(sup 1)-acetyl-
59. Acetamide, N-((4-aminophenyl)sulfonyl)-
60. Acetamide, N-[(4-aminophenyl)sulfonyl]-
61. Nsc 63871
62. N-(p-aminobenzenesulfonyl)acetamide
63. N(1)-acetylsulfanilamide
64. Mfcd00066501
65. Nsc-63871
66. Chembl455
67. Triple Sulfa (sulfacetamide)
68. Metimyd
69. Mls000069710
70. Solfacetamide
71. Chebi:63845
72. Ocusulf-30
73. Bleph-30
74. N-acetyl-4-amino-benzenesulfonamide
75. N(1)-acetyl-4-aminophenylsulfonamide
76. 4965g3j0f5
77. Sulfacyl (van)
78. Ncgc00018242-04
79. Ncgc00018242-07
80. Smr000058173
81. N1-acetylsulfanilamide
82. Solfacetamide [dcit]
83. Sulfacetamide 100 Microg/ml In Acetonitrile
84. Dsstox_cid_6060
85. 5414-91-5
86. Dsstox_rid_78000
87. Sulfanilazetamid [german]
88. Dsstox_gsid_26060
89. N-acetylsulfanilamine
90. Sulfacetamidum [inn-latin]
91. Sulfacetamida [inn-spanish]
92. N(sup 1)-acetylsulfanilamide
93. Caswell No. 808a
94. Cas-144-80-9
95. Ccris 6273
96. Einecs 205-640-6
97. Sulfacetamide (usp/inn)
98. Epa Pesticide Chemical Code 077904
99. Brn 0981718
100. Ai3-26837
101. Sulfacetamide [usp:inn:ban]
102. Unii-4965g3j0f5
103. Sulfacetamide, 1
104. Cetapred
105. Predamide
106. Spectrum_000984
107. Opera_id_1990
108. Prestwick0_000014
109. Prestwick1_000014
110. Prestwick2_000014
111. Prestwick3_000014
112. Spectrum2_001318
113. Spectrum3_001017
114. Spectrum4_001146
115. Spectrum5_001230
116. Wln: Zr Dswmv1
117. Sulfacetamide [mi]
118. Cbchromo1_000180
119. Sulfanilamide, N1-acetyl-
120. 4-acetylaminosulfonylaniline
121. Sulfacetamide [inn]
122. Nciopen2_002838
123. Sulfacetamide, >=98.0%
124. Oprea1_686524
125. Oprea1_810285
126. Schembl40863
127. Bspbio_000047
128. Bspbio_002773
129. Cbdive_013934
130. Kbiogr_001691
131. Kbioss_001464
132. Sulfacetamide [vandf]
133. 4-14-00-02662 (beilstein Handbook Reference)
134. Mls001076499
135. Divk1c_000227
136. Spectrum1500545
137. Sulfacetamide [mart.]
138. Spbio_001415
139. Spbio_001968
140. Sulfacetamide [who-dd]
141. Sulfacetamide [who-ip]
142. Bpbio1_000053
143. Dtxsid8026060
144. Hms500l09
145. Kbio1_000227
146. Kbio2_001464
147. Kbio2_004032
148. Kbio2_006600
149. Kbio3_001993
150. 4-[(acetylamino)sulfonyl]aniline
151. Ninds_000227
152. 4-[(acetylamino) Sulfonyl]aniline
153. Hms1921a11
154. Hms2092i13
155. Hms2232k07
156. Hms3259g06
157. Hms3374o02
158. Pharmakon1600-01500545
159. Sulfacetamide [orange Book]
160. Sulfacetamide, >=98.0% (nt)
161. Hy-n7123
162. Nsc63871
163. Zinc5179119
164. Sulfacetamide (triple Sulfa)
165. Sulfacetamide [usp Impurity]
166. Tox21_110846
167. Tox21_201999
168. Tox21_303100
169. Ac2473
170. Bbl012083
171. Bdbm50316126
172. Ccg-39256
173. Nsc757323
174. S5546
175. Stk068185
176. Trysul Component Sulfacetamide
177. Sulfacetamidum [who-ip Latin]
178. Sultrin Component Sulfacetamide
179. Vagilia Component Sulfacetamide
180. Akos000119729
181. N-[(4-aminobenzene)sulfonyl]acetamide
182. Tox21_110846_1
183. Ac-7630
184. Db00634
185. Ks-5327
186. Nc00538
187. Nsc-757323
188. Gyne-sulf Component Sulfacetamide
189. Idi1_000227
190. Sulfacetamide Component Of Trysul
191. Ncgc00018242-01
192. Ncgc00018242-02
193. Ncgc00018242-03
194. Ncgc00018242-05
195. Ncgc00018242-06
196. Ncgc00018242-08
197. Ncgc00021857-03
198. Ncgc00021857-04
199. Ncgc00021857-05
200. Ncgc00257159-01
201. Ncgc00259548-01
202. Sulfacetamide 100 Microg/ml In Methanol
203. Sulfacetamide Component Of Sultrin
204. Sulfacetamide Component Of Vagilia
205. Sy014752
206. Sbi-0051519.p003
207. Db-042749
208. Sulfacetamide Component Of Gyne-sulf
209. Cs-0013812
210. Ft-0631838
211. S0577
212. Sulfadimidine Impurity E [ep Impurity]
213. D05947
214. Ab00052094_14
215. Sr-01000000194
216. Sulfacetamide, Vetranal(tm), Analytical Standard
217. Triple Sulfa (sulfacetamide) [orange Book]
218. Q-201757
219. Q2022983
220. Sr-01000000194-2
221. Sulfacetamide, Antibiotic For Culture Media Use Only
222. Brd-k21520694-001-12-3
223. N-((4-aminophenyl)sulfonyl)acetamide [who-ip]
224. Sulfacetamide, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 214.24 g/mol |
|---|---|
| Molecular Formula | C8H10N2O3S |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 214.04121336 g/mol |
| Monoisotopic Mass | 214.04121336 g/mol |
| Topological Polar Surface Area | 97.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 299 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Bleph-10 |
| PubMed Health | Sulfacetamide |
| Drug Classes | Antiacne Antibacterial, Antibiotic, Antiseborrheic, Dermatological Agent |
| Drug Label | BLEPH-10 (sulfacetamide sodium ophthalmic solution, USP) 10% is a sterile, topical anti-bacterial agent for ophthalmic use. The active ingredient is represented by the following structural formula:... |
| Active Ingredient | Sulfacetamide sodium |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 10% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 6 | |
|---|---|
| Drug Name | Cetamide |
| PubMed Health | Sulfacetamide/Sulfur (On the skin) |
| Drug Classes | Antiacne, Antiacne Antibacterial, Antibacterial, Antibacterial Cleansing Agent, Antiseborrheic, Keratolytic |
| Drug Label | Each gram contains 100 mg of sodium sulfacetamide in a vehicle consisting of: cocamidopropyl betaine, disodium EDTA, methylparaben, PEG-60 almond triglycerides, PEG-150 pentaerythrityl tetrastearate (and) aqua (and) PEG-6 caprylic/capric glycerides,... |
| Active Ingredient | Sulfacetamide sodium |
| Dosage Form | Ointment |
| Route | Ophthalmic |
| Strength | 10% |
| Market Status | Prescription |
| Company | Alcon |
| 3 of 6 | |
|---|---|
| Drug Name | Klaron |
| PubMed Health | Sulfacetamide |
| Drug Classes | Antiacne Antibacterial, Antibiotic, Antiseborrheic, Dermatological Agent |
| Drug Label | Each mL of Klaron (sodium sulfacetamide lotion) Lotion, 10% contains 100 mg of sodium sulfacetamide in a vehicle consisting of purified water; propylene glycol; lauramide DEA (and) diethanolamine; polyethylene glycol 400, monolaurate; hydroxyethyl... |
| Active Ingredient | Sulfacetamide sodium |
| Dosage Form | Lotion |
| Route | Topical |
| Strength | 10% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 4 of 6 | |
|---|---|
| Drug Name | Bleph-10 |
| PubMed Health | Sulfacetamide |
| Drug Classes | Antiacne Antibacterial, Antibiotic, Antiseborrheic, Dermatological Agent |
| Drug Label | BLEPH-10 (sulfacetamide sodium ophthalmic solution, USP) 10% is a sterile, topical anti-bacterial agent for ophthalmic use. The active ingredient is represented by the following structural formula:... |
| Active Ingredient | Sulfacetamide sodium |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 10% |
| Market Status | Prescription |
| Company | Allergan |
| 5 of 6 | |
|---|---|
| Drug Name | Cetamide |
| PubMed Health | Sulfacetamide/Sulfur (On the skin) |
| Drug Classes | Antiacne, Antiacne Antibacterial, Antibacterial, Antibacterial Cleansing Agent, Antiseborrheic, Keratolytic |
| Drug Label | Each gram contains 100 mg of sodium sulfacetamide in a vehicle consisting of: cocamidopropyl betaine, disodium EDTA, methylparaben, PEG-60 almond triglycerides, PEG-150 pentaerythrityl tetrastearate (and) aqua (and) PEG-6 caprylic/capric glycerides,... |
| Active Ingredient | Sulfacetamide sodium |
| Dosage Form | Ointment |
| Route | Ophthalmic |
| Strength | 10% |
| Market Status | Prescription |
| Company | Alcon |
| 6 of 6 | |
|---|---|
| Drug Name | Klaron |
| PubMed Health | Sulfacetamide |
| Drug Classes | Antiacne Antibacterial, Antibiotic, Antiseborrheic, Dermatological Agent |
| Drug Label | Each mL of Klaron (sodium sulfacetamide lotion) Lotion, 10% contains 100 mg of sodium sulfacetamide in a vehicle consisting of purified water; propylene glycol; lauramide DEA (and) diethanolamine; polyethylene glycol 400, monolaurate; hydroxyethyl... |
| Active Ingredient | Sulfacetamide sodium |
| Dosage Form | Lotion |
| Route | Topical |
| Strength | 10% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
For the treatment of bacterial vaginitis, keratitis, acute conjunctivitis, and blepharitis.
FDA Label
Sulfacetamide is a sulfonamide antibiotic with bacteriostatic actions and broad-spectrum activity against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AF - Antiinfectives for treatment of acne
D10AF06 - Sulfacetamide
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AB - Sulfonamides
S01AB04 - Sulfacetamide
7-12.8 hours
Sulfacetamide is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), an essential component for bacterial growth (according to the Woods-Fildes theory). The inhibited reaction is necessary in these organisms for the synthesis of folic acid.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
12
PharmaCompass offers a list of Sulfacetamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sulfacetamide manufacturer or Sulfacetamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sulfacetamide manufacturer or Sulfacetamide supplier.
PharmaCompass also assists you with knowing the Sulfacetamide API Price utilized in the formulation of products. Sulfacetamide API Price is not always fixed or binding as the Sulfacetamide Price is obtained through a variety of data sources. The Sulfacetamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sulfacetamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sulfacetamide, including repackagers and relabelers. The FDA regulates Sulfacetamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sulfacetamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sulfacetamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sulfacetamide supplier is an individual or a company that provides Sulfacetamide active pharmaceutical ingredient (API) or Sulfacetamide finished formulations upon request. The Sulfacetamide suppliers may include Sulfacetamide API manufacturers, exporters, distributors and traders.
click here to find a list of Sulfacetamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sulfacetamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Sulfacetamide active pharmaceutical ingredient (API) in detail. Different forms of Sulfacetamide DMFs exist exist since differing nations have different regulations, such as Sulfacetamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sulfacetamide DMF submitted to regulatory agencies in the US is known as a USDMF. Sulfacetamide USDMF includes data on Sulfacetamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sulfacetamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sulfacetamide suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sulfacetamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sulfacetamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sulfacetamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sulfacetamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sulfacetamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sulfacetamide suppliers with NDC on PharmaCompass.
Sulfacetamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sulfacetamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sulfacetamide GMP manufacturer or Sulfacetamide GMP API supplier for your needs.
A Sulfacetamide CoA (Certificate of Analysis) is a formal document that attests to Sulfacetamide's compliance with Sulfacetamide specifications and serves as a tool for batch-level quality control.
Sulfacetamide CoA mostly includes findings from lab analyses of a specific batch. For each Sulfacetamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sulfacetamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Sulfacetamide EP), Sulfacetamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sulfacetamide USP).
