
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
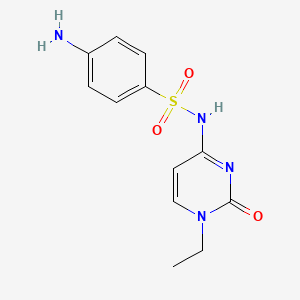

1. N(1)-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)sulfanilamide
1. Renoquid
2. Sulfacitine
3. 17784-12-2
4. 1-ethyl-n-sulfanilylcytosine
5. 1-ethyl N4-sulfanilylcytosin
6. Sulfacitinum [inn-latin]
7. Sulfacitina [inn-spanish]
8. Ci-636
9. N-sulfanilyl-l-ethylcytosine
10. 1401-49-6
11. N-sulfanilyl-1-ethylcytosine
12. 4-amino-n-(1-ethyl-2-oxopyrimidin-4-yl)benzenesulfonamide
13. Nsc 356717
14. Sulfacitine [inn]
15. Sulfacytine (usan)
16. Renoquid; Sulfacitine
17. Cl 636
18. Sulfanilamide, N1-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-
19. Nsc-356717
20. N(sup 1)-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)sulfanilamide
21. 4-amino-n-(1-ethyl-2-oxo-1,2-dihydropyrimidin-4-yl)benzenesulfonamide
22. Benzenesulfonamide, 4-amino-n-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-
23. T795873ajp
24. Sulfacitina
25. Sulfacitinum
26. Sulfacytine [usan]
27. Nl-sulfanilyl-1-ethylcytosine
28. Benzenesulfonamide,4-amino-n-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-
29. Renoquid (tn)
30. Hsdb 3272
31. Sulfactin (antibiotic)
32. Ci 636
33. Unii-t795873ajp
34. Sulfacytine [usan:inn:ban]
35. Starbld0009659
36. Sulfacytine [mi]
37. Sulfanilamide, N(1)-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-
38. Dsstox_cid_3606
39. Sulfacytine [hsdb]
40. Sulfacytine [vandf]
41. Dsstox_rid_97550
42. Sulfacytine [mart.]
43. Dsstox_gsid_23606
44. Schembl49377
45. Sulfacitine [who-dd]
46. Zinc2092
47. Chembl1201056
48. Dtxsid6023606
49. Sulfacytine [orange Book]
50. Chebi:135230
51. N(sup1)-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)sulfanilamide
52. Tox21_113734
53. Nsc356717
54. Db01298
55. Ncgc00253598-01
56. Da-09179
57. Hy-16472
58. Cas-17784-12-2
59. Cs-0006353
60. Ft-0710995
61. D02519
62. Sulfanilamide,2-dihydro-2-oxo-4-pyrimidinyl)-
63. Benzenesulfonamide,2-dihydro-2-oxo-4-pyrimidinyl)-
64. Q7636169
65. N1-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)sulfanilamide
66. 4-amino-n-(1-ethyl-2-oxo-1,2-dihydro-4-pyrimidinyl)benzenesulfonamide #
67. 4-amino-n-(1-ethyl-2-oxo-1,2-dihydropyrimidin-4-yl)benzene-1-sulfonamide
| Molecular Weight | 294.33 g/mol |
|---|---|
| Molecular Formula | C12H14N4O3S |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 294.07866149 g/mol |
| Monoisotopic Mass | 294.07866149 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 527 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents /SRP: Antibacterial/
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Sulfacytine is used to treat acute urinary tract infections caused by susceptible strains of Escherichia coli, the Klebsiella-Enterobacter group, Staphylococcus aureus, Proteus mirabilis, & less frequently, Proteus vulgaris.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
Sulfonamides are indicated in the treatment of chancroid caused by Hemophilus ducreyi. However, other agents such as erythromycin and ceftriaxone, are considered to be first line agents. /Sulfonamides; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2660
Sulfonamides are indicated in the treatment of endocervical and urethral infections caused by Chlamydia trachomatis. However, other agents, such as doxycycline and azithromycin, are considered to be first line agents. /Sulfonamides; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2660
For more Therapeutic Uses (Complete) data for SULFACYTINE (15 total), please visit the HSDB record page.
The number of conditions for which the sulfonamides are therapeutically useful and constitute drugs of first choice has been reduced sharply by the development of more effective antimicrobial agents and by the gradual increase in the resistance of a number of bacterial species to this class of drugs. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1062
Although the risk of crystalluria apparently is minimal, fluid intake should be increased when this drug is prescribed. Sulfacytine should be used with caution in patients with impaired renal function.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
...Because no well-controlled clinical studies exist.../sulfacytine/ should not be used during pregnancy unless the expected benefits outweigh the possible adverse effects of the drug.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
Sulfacytine is contraindicated in individuals allergic to sulfonamides.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
For more Drug Warnings (Complete) data for SULFACYTINE (26 total), please visit the HSDB record page.
Used orally in the treatment of acute urinary tract infections.
Sulfacytine is a short-acting sulfonamide. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Absorption
Well absorbed following oral administration.
Sulfacytine...is rapidly absorded following oral administration. More than 90% is excreted by kidneys almost entirely in the free, active form. ...86% is bound to serum proteins. ...The drug crosses the placenta & is excreted in milk...
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
By comparison to sulfisoxazole urine levels, 1 g/day of sulfacytine appears to be appropriate therapeutic dose & produces urine concn at least 10 times highest min inhibitory concentration found for sensitive microorganisms.
SMITH TC ET AL; COMPARATIVE URINE CONCN OF SULFACYTINE, A NEW SULFONAMIDE; J INT MED RES 1 (2) (1973)
Sulfacytine is highly soluble in urine within the normal acidic pH range.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
Thirty-four subjects were divided into 3 groups of 12, 12, and 10 respectively. The first group received 250 mg /sulfacytine/ 4 times a day, the second, 500 mg 4 times a day, and the third group received placebo. The renal function was not altered during the 84 days of the trial. Creatinine clearance, urea nitrogen, urinalysis, and phenosulfophthalein excretion tests were performed to evaluate kidney function.
PMID:4483531 Moyer C et al; J Clin Pharmacol New Drugs 12: 254-258 (1972)
For more Absorption, Distribution and Excretion (Complete) data for SULFACYTINE (11 total), please visit the HSDB record page.
Sulfonamides undergo metabolic alterations to varying extent in tissues, especially in liver. Both acetylation & oxidation occur. ... In nearly all species, major metabolic derivative is N4-acetylated sulfonamide. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1059
Although the liver is the major site of metabolism, sulfonamides may also be metabolized in other body tissues. Most sulfonamides are metabolized mainly by N4-acetylation. The degree of acetylation, which is a function of time, varies from less than 5% for sulfamethizole to up to 40% for sulfadiazine. The N4-acetyl metabolites, which do not possess antibacterial activity, have greater affinity for plasma albumin than does the nonacetylated drug and are usually less soluble than the parent sulfonamide, particularly in acidic urine. Like acetyl derivatives, glucuronide derivatives do not possess antibacterial activity; however, glucuronide derivatives are water soluble, appear to resemble the nonacetylated sulfonamide in plasma binding capacity, and have not been associated with adverse effects. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Sulfacytine has a biological half-life of approximately 4 hrs...
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1310
Sulfacytine is a competitive inhibitor of the enzyme dihydropteroate synthetase. It inhibits bacterial synthesis of of dihydrofolic acid by preventing the condensation of the pteridine with para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.
Sulfonamides are usually bacteriostatic in action. Sulfonamides interfere with the utilization of p-aminobenzoic acid (PABA) in the biosynthesis of tetrahydrofolic acid (the reduced form of folic acid) cofactors in susceptible bacteria. Sulfonamides are structural analogs of PABA and appear to interfere with PABA utilization by competitively inhibiting the enzyme dihydropteroate synthase, which catalyzes the formation of dihydropteroic acid (a precursor of tetrahydrofolic acid) from PABA and pteridine; however, other mechanism(s) affecting the biosynthetic pathway also may be involved. Compounds such as pyrimethamine and trimethoprim, which block later stages in the synthesis of folic acid, act synergistically with sulfonamides. Only microorganisms that synthesize their own folic acid are inhibited by sulfonamides; animal cells and bacteria which are capable of utilizing folic acid precursors or preformed folic acid are not affected by these drugs. The antibacterial activity of the sulfonamides is reportedly decreased in the presence of blood or purulent body exudates. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 424
The sulfonamides are structural analogs of para-aminobenzoic acid (PABA) and competitively inhibit an enzymatic step (dihydropterate synthetase) during which PABA is incorporated into the synthesis of dihydrofolic acid (folic acid). Because dihydrofolate synthesis is reduced, the levels of tetrahydrofolate (folinic acid) formed from dihydrofolate diminish. Tetrahydrofolate is an essential component of the coenzymes responsible for single carbon metabolism in cells. Acting as antimetabolites to PABA, sulfonamides eventually block, in a complex fashion, several enzymes. These enzymes include those needed for the biogenesis of purine bases; for the transfer of desoxyuridine to thymidine; and for the biosynthesis of methionine, glycine, and formylmethionyl-transfer-RNA. This results in suppression of protein synthesis, impairment of metabolic processes, and inhibition of growth and multiplication of those organisms that cannot use preformed folate. The effect is bacteriostatic, although a bactericidal action is evident at the high concentrations that may be found in urine.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2076
ABOUT THIS PAGE
85
PharmaCompass offers a list of Sulfacytine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sulfacytine manufacturer or Sulfacytine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sulfacytine manufacturer or Sulfacytine supplier.
PharmaCompass also assists you with knowing the Sulfacytine API Price utilized in the formulation of products. Sulfacytine API Price is not always fixed or binding as the Sulfacytine Price is obtained through a variety of data sources. The Sulfacytine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sulfacytine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sulfacytine, including repackagers and relabelers. The FDA regulates Sulfacytine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sulfacytine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Sulfacytine supplier is an individual or a company that provides Sulfacytine active pharmaceutical ingredient (API) or Sulfacytine finished formulations upon request. The Sulfacytine suppliers may include Sulfacytine API manufacturers, exporters, distributors and traders.
Sulfacytine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sulfacytine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sulfacytine GMP manufacturer or Sulfacytine GMP API supplier for your needs.
A Sulfacytine CoA (Certificate of Analysis) is a formal document that attests to Sulfacytine's compliance with Sulfacytine specifications and serves as a tool for batch-level quality control.
Sulfacytine CoA mostly includes findings from lab analyses of a specific batch. For each Sulfacytine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sulfacytine may be tested according to a variety of international standards, such as European Pharmacopoeia (Sulfacytine EP), Sulfacytine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sulfacytine USP).
