
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
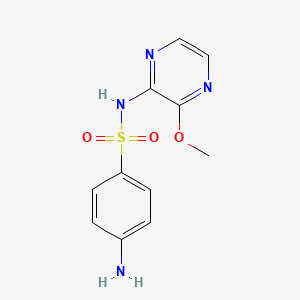

1. Kelfizine
2. Longum
3. Sulfamethopyrazine
4. Sulfamethoxypyrazine
5. Sulfametopyrazine
6. Sulfapyrazinmethoxine
7. Sulphalene
8. Sulphametopyrazine
1. 152-47-6
2. Sulfamethoxypyrazine
3. Sulfametopyrazine
4. Sulfamethopyrazine
5. Sulfalen
6. 4-amino-n-(3-methoxypyrazin-2-yl)benzenesulfonamide
7. Kelfizine
8. Longum
9. Sulfapyrazinemethoxine
10. Sulfapyrazinemethoxyne
11. Kelfizin
12. Kelfizina
13. Polycidal
14. Dalysep
15. Sulfapyrazinemethoxyine
16. Kelfizine W
17. Smp2
18. 3-methoxy-2-sulfapyrazine
19. 2-sulfanilamido-3-methoxypyrazine
20. 3-methoxy-2-sulfanilamidopyrazine
21. Farmitalia 204/122
22. 3-methoxypyrazine Sulfanilamide
23. 2-methoxy-3-sulfanilamidopyrazine
24. Nsc-110433
25. N1-(3-methoxypyrazinyl)sulfanilamide
26. Benzenesulfonamide, 4-amino-n-(3-methoxypyrazinyl)-
27. 2-(p-aminobenzenesulfanamide)-3-methoxypyrazine
28. 2-(p-aminobenzenesulfonamido)-3-methoxypyrazine
29. As 18908
30. F.i. 5978
31. Pyrazine, 2-sulfanilamido-3-methoxy-
32. 4-amino-n-(3-methoxypyrazinyl)benzenesulfonamide
33. N1-(3-methoxy-2-pyrazinyl)sulfanilamide
34. N(sup1)-(3-methoxypyrazinyl)sulfanilamide
35. N(sup 1)-(3-methoxypyrazinyl)sulfanilamide
36. 4-amino-n-(3-methoxy-2-pyrazinyl)-benzenesulfonamide
37. Sulfalene(smpz)
38. N(sup1)-(3-methoxy-2-pyrazinyl)sulfanilamide
39. Sulfalene (usan)
40. N(sup 1)-(3-methoxy-2-pyrazinyl)sulfanilamide
41. As-18908
42. Sulfanilamide, N'-(3-methoxypyrazinyl)-
43. Sulfanilamide, N1-(3-methoxypyrazinyl)-
44. Sulfamethopyrazine (jan)
45. Sulfanilamide, N(sup 1)-(3-methoxypyrazinyl)-
46. Sulfanilamide, N(sup 1)-(3-methoxy-2-pyrazinyl)-
47. T6bl4zc15g
48. Sulfametopyrazine;as-18908
49. Sulfalenum
50. Chebi:32162
51. Solfametopirazina
52. Nsc110433
53. Sulfalene [usan:inn]
54. 4-amino-n-(3-methoxypyrazin-2-yl)benzene-1-sulfonamide
55. Sulfalenum [inn-latin]
56. Solfametopirazina [dcit]
57. Sulfaleno [inn-spanish]
58. Ncgc00160482-01
59. Sulfaleno
60. Sulfalene [usan]
61. Dsstox_cid_26179
62. Dsstox_rid_81410
63. Dsstox_gsid_46179
64. Sulfametoxypyridazin
65. 2-sulfanilamide 3-methoxy-pyrazine
66. Sulfamethopyrazine [jan]
67. 4-amino-n-(3-methoxy-pyrazin-2-yl)-benzenesulfonamide
68. Kelfizina (tn)
69. Cas-152-47-6
70. Smr000011031
71. Fi 5978
72. Wr 4629
73. Einecs 205-804-7
74. Cbmicro_013257
75. Unii-t6bl4zc15g
76. Nsc 110433
77. Brn 0622512
78. Vetkelfizina
79. Policydal
80. N-(3-methoxypyrazin-2-yl)sulfanilamide
81. Sulfalene [inn]
82. Sulfalene [mi]
83. Sulfalene [who-dd]
84. Oprea1_010520
85. Schembl28404
86. 4-amino-n-(3-methoxypyrazinyl)-benzenesulfonamide
87. 5-25-12-00574 (beilstein Handbook Reference)
88. Mls001304101
89. Mls001304170
90. Butadiene Sulfone;3-sulfolene
91. 4-amino-n-(3-methoxypyrazin-2-yl);benzenesulfonamide
92. Zinc2097
93. Sulfalene (sulfamethoxypyrazine)
94. Chembl1525826
95. Dtxsid2046179
96. Gtpl10174
97. Sulfametopyrazine [mart.]
98. Hms2866d06
99. Hms3604l11
100. Bcp30869
101. Hy-a0130
102. Smsf0004129
103. Tox21_111844
104. Wln: T6n Dnj Bo1 Cmswr Dz
105. Ac-750
106. Bbl023067
107. Mfcd00437754
108. S4976
109. Stk386859
110. Akos000538590
111. Tox21_111844_1
112. Cb03172
113. Ccg-112228
114. Cs-8165
115. Db00664
116. Ks-5333
117. Ncgc00160482-02
118. Sulfalene 100 Microg/ml In Acetonitrile
119. Bim-0013167.p001
120. Db-043149
121. Ft-0603363
122. Sulfanilamide, N1-(3-methoxy-2-pyrazinyl)-
123. A16975
124. D01216
125. Sulfalene Sulfamethoxypyrazine Sulfametopyrazine
126. 152s476
127. A809315
128. Sr-01000817374
129. Q-201763
130. Q6577302
131. Sr-01000817374-3
132. Z277559098
133. 4-amino-n-(3-methoxypyrazin-2-yl)benzenesulfonamide;sulfalene
134. Sulfametopyrazine; As-18908; Sulfalene; Sulfaleno;sulfamethoxypyrazine
135. 5175-55-3
| Molecular Weight | 280.31 g/mol |
|---|---|
| Molecular Formula | C11H12N4O3S |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 280.06301143 g/mol |
| Monoisotopic Mass | 280.06301143 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 376 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of urinary tract infection and chronic bronchitis.
Sulfametopyrazine is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01ED - Long-acting sulfonamides
J01ED02 - Sulfalene
Sulfametopyrazine is a competitive inhibitor of the bacterial enzyme dihydropteroate synthetase. Para-aminobenzoic acid (PABA), a substrate of the enzyme is prevented from binding. The inhibited reaction is necessary in these organisms for the synthesis of folic acid.
Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?