
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
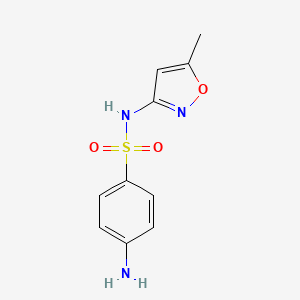

1. Gantanol
2. Sulfamethylisoxazole
3. Sulfisomezole
4. Sulphamethoxazole
1. 723-46-6
2. Gantanol
3. Sulphamethoxazole
4. Sulfisomezole
5. Sulfamethoxazol
6. Metoxal
7. 4-amino-n-(5-methyl-3-isoxazolyl)benzenesulfonamide
8. Sulfamethylisoxazole
9. Simsinomin
10. Radonil
11. Sinomin
12. Sulphamethoxazol
13. 4-amino-n-(5-methylisoxazol-3-yl)benzenesulfonamide
14. Sulpha-methoxizole
15. Bactrim
16. Sulfamethalazole
17. Azo-gantanol
18. Sulphamethylisoxazole
19. Ro 4-2130
20. Urobak
21. 3-sulfanilamido-5-methylisoxazole
22. Gantanol-ds
23. 4-amino-n-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide
24. Sulfamethoxazolum
25. Sulphisomezole
26. Benzenesulfonamide, 4-amino-n-(5-methyl-3-isoxazolyl)-
27. 5-methyl-3-sulfanilamidoisoxazole
28. Ms 53
29. 3-sulphanilamido-5-methylisoxazole
30. 5-methyl-3-sulphanil-amidoisoxazole
31. 3-(p-aminophenylsulfonamido)-5-methylisoxazole
32. 5-methyl-3-sulfanylamidoisoxazole
33. N'-(5-methyl-3-isoxazolyl)sulfanilamide
34. N1-(5-methyl-3-isoxazolyl)sulfanilamide
35. N'-(5-methylisoxazol-3-yl)sulphanilamide
36. 3-(para-aminophenylsulphonamido)-5-methylisoxazole
37. Sulmeprim
38. Smx
39. Trimeth/sulfa
40. A047
41. Sulfanilamide, N1-(5-methyl-3-isoxazolyl)-
42. N(sup 1)-(5-methyl-3-isoxazolyl)sulphanilamide
43. Chebi:9332
44. N'-(5-methyl-3-isoxazole)sulfanilamide
45. Sulfamethoxizole
46. Ro 6-2580/11
47. Stx-608
48. 4-amino-n-(5-methyl-isoxazol-3-yl)-benzenesulfonamide
49. Sulfanilamide, N'-(5-methyl-3-isoxazolyl)-
50. 4-amino-n-(5-methyl-3-isoxazoyl)benzenesulfonamide
51. Bactrimel
52. Gamazole
53. Mfcd00010546
54. N(sup 1)-(5-methyl-3-isoxazolyl)sulfanilamide
55. Chembl443
56. Nsc-147832
57. Ro-4-2130
58. Mls000069732
59. Je42381tnv
60. Sulfametoxazol
61. 4-amino-n-(5-methyl-1,2-oxazol-3-yl)benzene-1-sulfonamide
62. Solfametossazolo
63. N(sup1)-(5-methyl-3-isoxazolyl)sulfanilamide
64. 5-methyl-3-sulfonylamidoisoxazole
65. 3-(p-aminobenzenesulfonamido)-5-methylisoxazole
66. Nsc147832
67. 4-amino-n-(5-methyl-3-isoxazolyl)-benzenesulfonamide
68. 144930-01-8
69. Ncgc00016533-05
70. Ncgc00186654-01
71. Cas-723-46-6
72. Smr000058223
73. Dsstox_cid_6064
74. Sulfamethoxazole 100 Microg/ml In Acetonitrile
75. Solfametossazolo [dcit]
76. Dsstox_rid_78001
77. Dsstox_gsid_26064
78. 3-methyl-1-(4-sulfoamidophenyl)-5-pyra&
79. Sulfametoxazol [inn-spanish]
80. Sulfamethoxazolum [inn-latin]
81. Benzenesulfonamide, 4-amino-n-(5-methyl-3-isoxazolyl)-, Radical Ion(1+)
82. Ccris 567
83. Hsdb 3186
84. Sr-01000000217
85. Einecs 211-963-3
86. Nsc 147832
87. Brn 0226453
88. Unii-je42381tnv
89. Sulfanilamide, N(1)-(5-methyl-3-isoxazolyl)-
90. 08d
91. Prestwick_453
92. Albb-002089
93. Septran (salt/mix)
94. Septrin (salt/mix)
95. Sulfamethoxazole,(s)
96. Eusaprim (salt/mix)
97. Spectrum_000994
98. Sulfamethoxazole(usan)
99. Starbld0000281
100. Opera_id_882
101. Maybridge1_007190
102. Prestwick0_000177
103. Prestwick1_000177
104. Prestwick2_000177
105. Prestwick3_000177
106. Spectrum2_000788
107. Spectrum3_000584
108. Spectrum4_000345
109. Spectrum5_000982
110. Sulfamethoxazole [usan:usp:inn:ban:jan]
111. Epitope Id:114999
112. Co-trimoxazole (salt/mix)
113. Schembl3656
114. Oprea1_114486
115. Oprea1_285680
116. Bspbio_000073
117. Bspbio_002028
118. Kbiogr_000749
119. Kbioss_001474
120. Sulfamethoxazole [mi]
121. Mls001055354
122. Mls001074165
123. Mls006011871
124. Bidd:gt0731
125. Divk1c_000649
126. Spectrum1500550
127. Sulfamethoxazole [inn]
128. Sulfamethoxazole [jan]
129. Spbio_000896
130. Spbio_001994
131. Sulfamethoxazole [hsdb]
132. Sulfamethoxazole [iarc]
133. Sulfamethoxazole [usan]
134. [(4-aminophenyl)sulfonyl](5-methylisoxazol-3-yl)amine
135. Bpbio1_000081
136. Sulfamethoxazole [vandf]
137. Dtxsid8026064
138. Sulfamethoxazole [mart.]
139. Component Of Bactrim (salt/mix)
140. Gtpl10933
141. Hms502a11
142. Hms561o18
143. Jlkigftwxxrpmt-uhfffaoysa-
144. Kbio1_000649
145. Kbio2_001474
146. Kbio2_004042
147. Kbio2_006610
148. Kbio3_001528
149. Zinc89763
150. N1-(5-methylisoxazol-3-yl)-4-aminobenzene-1-sulfonamide
151. Stx 608
152. Sulfamethoxazole [usp-rs]
153. Sulfamethoxazole [who-dd]
154. Sulfamethoxazole [who-ip]
155. Wln: T5noj C1 Emswr Dz
156. Ninds_000649
157. Hms1568d15
158. Hms1921a21
159. Hms2092k03
160. Hms2095d15
161. Hms2233l13
162. Hms3259e06
163. Hms3372m22
164. Hms3655o22
165. Hms3712d15
166. Pharmakon1600-01500550
167. Bcp02881
168. Hy-b0322
169. Sulfamethoxazole (jp17/usp/inn)
170. Sulfamethoxazole, Analytical Standard
171. Tox21_110480
172. Tox21_200353
173. Bbl004554
174. Bdbm50029770
175. Ccg-40166
176. Nsc757328
177. Ro-42130
178. S1915
179. Stk007988
180. Sulfamethoxazole [orange Book]
181. Akos000200952
182. Component Of Azo Gantanol (salt/mix)
183. Tox21_110480_1
184. Bs-3542
185. Db01015
186. Nc00537
187. Nsc-757328
188. Rp-2145
189. Sulfamethoxazole [ep Monograph]
190. Cotrim Component Sulfamethoxazole
191. Idi1_000649
192. Septra Component Sulfamethoxazole
193. Sulfamethoxazole [usp Monograph]
194. Sulfamethoxazolum [who-ip Latin]
195. Bactrim Component Sulfamethoxazole
196. Ncgc00016533-01
197. Ncgc00016533-02
198. Ncgc00016533-03
199. Ncgc00016533-04
200. Ncgc00016533-06
201. Ncgc00016533-07
202. Ncgc00016533-08
203. Ncgc00016533-09
204. Ncgc00016533-10
205. Ncgc00016533-11
206. Ncgc00016533-12
207. Ncgc00016533-14
208. Ncgc00021995-03
209. Ncgc00021995-04
210. Ncgc00021995-05
211. Ncgc00257907-01
212. Uroplus Component Sulfamethoxazole
213. Ac-11118
214. Sy018888
215. Bcp0726000283
216. N'-(5-methyl-3-isoxazolyl)-sulfanilamide
217. N1-(5-methyl-3-isoxazolyl)-sulfanilamide
218. N1-(5-methyl-3-isoxazolyl)sulphanilamide
219. Sbi-0051524.p003
220. Sulfamethoxazole Component Of Cotrim
221. Sulfamethoxazole Component Of Septra
222. Sulfatrim Component Sulfamethoxazole
223. Sulmeprim Component Sulfamethoxazole
224. Bactrim Ds Component Sulfamethoxazole
225. Co-trimethoxazole Component Sulfamethoxazole
226. Db-055629
227. Sulfamethoxazole 100 Microg/ml In Methanol
228. Sulfamethoxazole Component Of Bactrim
229. Sulfamethoxazole Component Of Uroplus
230. Ab00052099
231. Bb 0242379
232. Ft-0602616
233. N^1-(5-methyl-3-isoxazolyl)-sulfanilamide
234. Sulfamethoxazole 1000 Microg/ml In Methanol
235. Sw196670-3
236. Azo Gantanol Component Sulfamethoxazole
237. Cotrim D.s. Component Sulfamethoxazole
238. Sulfamethoxazole Component Of Sulfatrim
239. Sulfamethoxazole Component Of Sulmeprim
240. C07315
241. D00447
242. F12027
243. Sulfamethoprim Component Sulfamethoxazole
244. Sulfamethoxazole Component Of Bactrim Ds
245. Ab00052099-14
246. Ab00052099-16
247. Ab00052099_17
248. Ab00052099_18
249. Sulfamethoxazole 1000 Microg/ml In Acetonitrile
250. Sulfamethoxazole Component Of Azo Gantanol
251. Sulfamethoxazole Component Of Cotrim D.s.
252. Bactrim Pediatric Component Sulfamethoxazole
253. Ndimethyl1-(5-methyl-3-isoxazolyl)-sulfanilamide
254. Q415843
255. Sulfamethoxazole Component Of Sulfamethoprim
256. Q-201762
257. Sr-01000000217-2
258. Sr-01000000217-3
259. Sulfamethoxazole, Vetranal(tm), Analytical Standard
260. 4-amino-n-(5-methylisoxazol-3-yl)benzenesulphonamide
261. Brd-k28494619-001-05-0
262. Brd-k28494619-001-15-9
263. Brd-k28494619-001-26-6
264. Sulfamethoxazole Component Of Bactrim Pediatric
265. Z57198677
266. 4-amino-n-(5-methyl-isoxazol-3-yl)-benzene Sulfonamide
267. Sulfamethoxazole, British Pharmacopoeia (bp) Reference Standard
268. Sulfamethoxazole, Certified Reference Material, Tracecert(r)
269. 4-amino-n-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide; Smx; Smz
270. Sulfamethoxazole, European Pharmacopoeia (ep) Reference Standard
271. Sulfamethoxazole, United States Pharmacopeia (usp) Reference Standard
272. Sulfamethoxazole, Pharmaceutical Secondary Standard; Certified Reference Material
273. 129378-89-8
| Molecular Weight | 253.28 g/mol |
|---|---|
| Molecular Formula | C10H11N3O3S |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 253.05211239 g/mol |
| Monoisotopic Mass | 253.05211239 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 346 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents /SRP: Antibacterial/
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Sulfamethoxazole is indicated/ for the treatment of urinary tract infections due to susceptible strains of the following organisms: Escherichia coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilis and Proteus vulgaris. It is recommended that initial episodes of uncomplicated urinary tract infections be treated with a single effective antibacterial agent rather than the combination. /Bactrim (sulfamethoxazole and trimethoprim); Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information. Bactrim (sulfamethoxazole and trimethoprim) (11/2006). Available from, as of July 09, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
/Sulfamethoxazole is indicated/ for the treatment of acute otitis media in pediatric patients due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when in the judgment of the physician sulfamethoxazole and trimethoprim offers some advantage over the use of other antimicrobial agents. To date, there are limited data on the safety of repeated use of BACTRIM in pediatric patients under two years of age. Bactrim is not indicated for prophylactic or prolonged administration in otitis media at any age. /Bactrim (sulfamethoxazole and trimethoprim); Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information. Bactrim (sulfamethoxazole and trimethoprim) (11/2006). Available from, as of July 09, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
For the treatment of acute exacerbations of chronic bronchitis due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when in the judgment of the physician Bactrim offers some advantage over the use of a single antimicrobial agent. /Bactrim (sulfamethoxazole and trimethoprim); Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information. Bactrim (sulfamethoxazole and trimethoprim) (11/2006). Available from, as of July 09, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
For more Therapeutic Uses (Complete) data for SULFAMETHOXAZOLE (24 total), please visit the HSDB record page.
The number of conditions for which the sulfonamides are therapeutically useful and constitute drugs of first choice has been reduced sharply by the development of more effective antimicrobial agents and by the gradual increase in the resistance of a number of bacterial species to this class of drugs. /Sulfonamides/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1062
Precautions must be observed to avoid sulfamethoxazole crystalluria because of high percentage of acetylated, relatively insoluble form of drug in urine.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1052
The leukopenic and thrombocytopenic effects of sulfonamides may result in an increased incidence of certain microbial infections, delayed healing, and gingival bleeding. If leukopenia or thrombocytopenia occurs, dental work should be deferred until blood counts have returned to normal. Patients should be instructed in proper oral hygiene, including caution in use of regular toothbrushes, dental floss, and toothpicks. /Sulfonamides/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2662
About 75% of the untoward effects involve the skin. ...trimethoprim-sulfamethoxazole has been reported to cause up to three times as many dermatological reactions as does sulfisoxazole when given alone. /co-trimoxazole/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1064
For more Drug Warnings (Complete) data for SULFAMETHOXAZOLE (27 total), please visit the HSDB record page.
Sulfamethoxazole is indicated in combination with trimethoprim, in various formulations, for the following infections caused by bacteria with documented susceptibility: urinary tract infections, acute otitis media in pediatric patients (when clinically indicated), acute exacerbations of chronic bronchitis in adults, enteritis caused by susceptible _Shigella_, prophylaxis and treatment of _Pneumocystis jiroveci_ pneumonia, and travelers' diarrhea caused by enterotoxigenic _E. coli_. In Canada, additional indications include the adjunctive treatment of cholera, treatment of bacillary dysentery, nocardiosis, and second-line treatment of brucellosis in combination with [gentamicin] or [rifampicin].
Sulfamethoxazole is a bacteriostatic sulfonamide antibiotic that inhibits a critical step in bacterial folate synthesis. It is generally given in combination with [trimethoprim], a dihydrofolate reductase inhibitor, which inhibits the reduction of dihydrofolic acid to tetrahydrofolic acid. Studies have shown that bacterial resistance develops more slowly with the combination of the two drugs than with either trimethoprim or sulfamethoxazole alone, as together they inhibit sequential steps in the bacterial folate synthesis pathway. Sulfonamides, including sulfamethoxazole, have been implicated in hypersensitivity reactions - these agents should be discontinued at the first sign of a developing rash, as this may signal the start of a more severe reaction such as Stevens-Johnson syndrome or toxic epidermal necrolysis. Sulfamethoxazole treatment may contribute to folate deficiency and should therefore be used with caution in patients at a higher risk of developing a deficiency. Hemolysis has been observed in patients with glucose-6-phosphate dehydrogenase deficiency who are using sulfamethoxazole/trimethoprim.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J01EE01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J01EC01
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J01EC01
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J01EC01
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J01EC01
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EC - Intermediate-acting sulfonamides
J01EC01 - Sulfamethoxazole
Absorption
Sulfamethoxazole is rapidly absorbed following oral administration and has a bioavailability of 85-90%. The Tmax is approximately 1-4 hours following oral administration, and the Cmax at steady-state is 57.4 - 68.0 g/mL.
Route of Elimination
Elimination occurs primarily via glomerular filtration and tubular secretion in the kidneys, with urine concentrations generally considerably higher than plasma concentrations. Approximately 84.5% of a single oral dose of sulfamethoxazole is recovered in the urine within 72 hours, of which ~30% is free sulfamethoxazole and the remainder is the N4-acetylated metabolite.
Volume of Distribution
The volume of distribution sulfamethoxazole following a single oral dose was found to be 13 L. Sulfamethoxazole distributes into sputum, vaginal fluid, middle ear fluid, breast milk, and the placenta.
Clearance
The oral and renal clearance of sulfamethoxazole have been estimated as 1.2 0.2 and 0.22 0.05 L/h, respectively.
In urine, approximately 20% of the sulfamethoxazole present is unchanged drug, 50-70% is the acetylated derivative, and 15-20% is the glucuronide conjugate.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 622
Sulfamethoxazole is widely distributed into most body tissues. Sulfamethoxazole crosses the placenta. In general, the total serum protein binding of sulfamethoxazole is considered to be 50-70%; the acetylated metabolite of sulfamethoxazole is protein bound to a somewhat greater extent than is the unmetabolized drug. In uremic patients, serum protein binding of sulfamethoxazole is reduced. Sulfamethoxazole has an apparent volume of 10-16 liters. In uremic patients, the apparent volume of distribution of sulfamethoxazole increases substantially; this increase is partly caused by reduced binding of the drug to serum proteins.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 622
Individual sulfonamides differ markedly in their absorption, distribution, and elimination. With the exception of sulfapyrimidine and sulfasalazine, which are only slightly absorbed, sulfonamides are generally well absorbed from the GI tract. Approximately 70-90% of an oral dose of the absorbable sulfonamides is reportedly absorbed from the small intestine; small amounts may also be absorbed from the stomach. Sulfamethizole and sulfisoxazole (no longer commercially available in the US) are absorbed rapidly; peak blood concentrations are usually obtained within 2-4 hours. Sulfadiazine and sulfapyridine are absorbed at a slower rate with peak blood concentrations occurring within 3-7 hours. Administration of oral sulfonamides with food appears to delay, but not reduce, absorption of the drugs. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
Absorption of sulfonamides from the vagina, respiratory tract, or abraded skin is variable and unreliable; however, enough drug may be absorbed to induce sensitization or toxicity. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
For more Absorption, Distribution and Excretion (Complete) data for SULFAMETHOXAZOLE (17 total), please visit the HSDB record page.
Sulfamethoxazole metabolism is mediated primarily by arylamine N-acetyltransferase (NAT) enzymes, which are responsible for acetylation of sulfamethoxazole at its N4 position. Sulfamethoxazole may also undergo oxidation at its C5 and N4 atoms, the latter of which is catalyzed by CYP2C9. Glucuronidation of the N4 atom, likely mediated by unspecified UGT enzymes, is an additional minor route of metabolism. None of the identified metabolites of sulfamethoxazole appear to carry antimicrobial activity. The hydroxylamine metabolite of sulfamethoxazole, generated via oxidation by CYP2C9, may be further converted to a more reactive nitroso- metabolite.
Although the liver is the major site of metabolism, sulfonamides may also be metabolized in other body tissues. Most sulfonamides are metabolized mainly by N4-acetylation. The degree of acetylation, which is a function of time, varies from less than 5% for sulfamethizole to up to 40% for sulfadiazine. The N4-acetyl metabolites, which do not possess antibacterial activity, have greater affinity for plasma albumin than does the nonacetylated drug and are usually less soluble than the parent sulfonamide, particularly in acidic urine. Like acetyl derivatives, glucuronide derivatives do not possess antibacterial activity; however, glucuronide derivatives are water soluble, appear to resemble the nonacetylated sulfonamide in plasma binding capacity, and have not been associated with adverse effects. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
The in vitro biotransformation of three sulfonamides ... was studied using primary cultures of pig hepatocytes. Incubation of monolayer cultures with sulfadimethoxine (SDM), sulfamethoxazole (SMZ) and 14C-sulfadimidine (SDD) resulted in the formation of the corresponding N4-acetylsulfonamide to different extents, depending upon the molecular structure of the drug. Addition of the acetylsulfonamides to the cells showed that these compounds were deacetylated, each to a different extent. A relatively low degree of acetylation (in case of 14C-sulfadimidine) was paralleled by extensive deacetylation (i.e. AcSDD), whereas extensive acetylation (i.e.sulfamethoxazole) was in concert with minor deacetylation (i.e.AcSMX). The addition of bovine serum albumin to the medium resulted in a decrease in conversion of sulfonamides as well as acetylsulfonamides. ...
PMID:9049946 Mengelers MJ et al; J Vet Pharmacol Ther 20 (10): 24-32 (1997)
The acetylation pattern of sulfamethoxazole was examined in six male and 16 female healthy volunteers selected according to their acetylation phenotype by analysis of the acetylation pattern of sulfadimidine. They were given a single oral dose of 10 mg/kg bw sulfamethoxazole, and blood (at 6 hr) and urine (0-6 hr) were analysed for the presence of total and free sulfamethoxazole (total minus free was considered to be the acetylated form). Sulfamethoxazole did not appear to undergo polymorphic acetylation.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 365
Acetylation of sulfamethoxazole by human hepatic monomorphic (NAT1) and polymorphic (NAT2) arylamine N-acetyltransferase showed a higher affinity for the monomorphic enzyme (Kmax, 1.2 mmol/L and approximately 5 mmol/L for NAT1 and NAT2, respectively). The higher affinity for NAT1 indicated that acetylation by this enzyme predominates at therapeutic plasma concentrations, in agreement with the observed monomorphic acetylation of sulfamethoxazole in vivo. There were no differences in affinity between human recombinant NAT1 and NAT2 enzymes in converting sulfamethoxazole hydroxylamine to the reactive N-acetoxysulfamethoxazole.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V79 366
For more Metabolism/Metabolites (Complete) data for SULFAMETHOXAZOLE (12 total), please visit the HSDB record page.
Sulfamethoxazole has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-[4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]anilino]oxane-2-carboxylic acid, Acetylsulfamethoxazole, and Sulfamethoxazole N4-hydroxylamine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The average serum half-life of sulfamethoxazole is 10 hours and may be increased in patients with severely impaired renal function.
Half life of sulfamethoxazole in babies during first 10 days of life is considerably longer than in adults. It falls rapidly, being about 9 hr at 3 wk of age and 4-5 hr at 1 yr. It then increases toward half life characteristic for adults, namely, 10-11 hr.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1060
In individuals with normal renal function, the elimination half-life of sulfamethoxazole is 7-12 hours. The elimination half-life of sulfamethoxazole begins to increase appreciably when the creatinine clearance rate decreases to about 30 mL/minute, and in patients with creatinine clearances of less than 10 mL/minute, a half-life of 22-50 hours has been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 622
Sulfonamides are generally classified as short-acting, intermediate-acting, or long-acting depending on the rate at which they are absorbed and eliminated. Sulfamethizole, sulfasalazine, and sulfisoxazole are generally considered to be short-acting sulfonamides and reportedly have plasma half-lives of about 4-8 hours. Sulfadiazine and sulfapyridine are generally considered to be intermediate-acting sulfonamides and reportedly have plasma half-lives of about 7-17 hours. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 425
The disposition kinetics of a single oral dose of sulfamethoxazole administered as a 960 mg dose of a combination product with trimethoprim (co-trimoxazole) were studied in 6 healthy male volunteers (aged 23 to 29 yr) and in 15 untreated pulmonary tuberculosis patients. The elimination half-life in healthy volunteers ranged from 8.67 to 13.7 hr and in patients, 6.5 to 13.5 hr. No significant difference in the elimination half-lives were found in healthy male volunteers and untreated patients with pulmonary tuberculosis.
Pandey S et al; East Pharm 33 (Feb): 121-3 (1990)
Sulfamethoxazole is a sulfonamide that inhibits bacterial dihydrofolic acid synthesis due to its structural similarity to an endogenous substrate, para-aminobenzoic acid (PABA). Most bacteria meet their need for folic acid by synthesizing it from PABA, as opposed to Animalia that require exogenous folic acid sources. Sulfamethoxazole competitively inhibits dihydropteroate synthase, the enzyme responsible for bacterial conversion of PABA to dihydrofolic acid. Inhibition of this pathway prevents the synthesis of tetrahydrofolate and, ultimately, the synthesis of bacterial purines and DNA, resulting in a bacteriostatic effect.
Sulfonamides are usually bacteriostatic in action. Sulfonamides interfere with the utilization of p-aminobenzoic acid (PABA) in the biosynthesis of tetrahydrofolic acid (the reduced form of folic acid) cofactors in susceptible bacteria. Sulfonamides are structural analogs of PABA and appear to interfere with PABA utilization by competitively inhibiting the enzyme dihydropteroate synthase, which catalyzes the formation of dihydropteroic acid (a precursor of tetrahydrofolic acid) from PABA and pteridine; however, other mechanism(s) affecting the biosynthetic pathway also may be involved. Compounds such as pyrimethamine and trimethoprim, which block later stages in the synthesis of folic acid, act synergistically with sulfonamides. Only microorganisms that synthesize their own folic acid are inhibited by sulfonamides; animal cells and bacteria which are capable of utilizing folic acid precursors or preformed folic acid are not affected by these drugs. The antibacterial activity of the sulfonamides is reportedly decreased in the presence of blood or purulent body exudates. /Sulfonamides/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 424
/Sulfonamides inhibit bacterial growth by preventing para-aminobenzoic acid (PABA) from being incorporated/ into dihydropteroic acid, the immediate precursor of folic acid. Sensitive microorganisms are those that must synthesize their own folic acid; bacteria that can utilize preformed folate are not affected. Bacteriostasis induced by sulfonamides is counteracted by PABA competitively. Sulfonamides do not affect mammalian cells by this mechanism, since they require preformed folic acid and cannot synthesize it. /Sulfonamides/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1048
Sulfonamides are broad-spectrum, bacteriostatic anti-infectives. They are structural analogs of para-aminobenzoic acid and competively inhibit a bacterial enzyme, dihydropteroate synthetase, that is responsible for incorporation of para-aminobenzoic acid into dihydrofolic acid. This blocks the synthesis of dihydrofolic acid and decreases the amount of metabolically active tetrahydrofolic acid, a cofactor for the synthesis of purines, thymidine, and DNA. /Sulfonamides/
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, V.IB p.2538 (1992)
The hydroxylamine and nitroso metabolites formed by N4-oxidation of sulfonamides are thought to be involved in the pathogenesis of idiosyncratic reactions to this class of drugs. Idiosyncratic reactions to sulfonamides are characterized by multisystemic toxicity, including hepatitis, nephritis, dermatitis, and blood dyscrasias (aplastic anemia, agranulocytosis). Previously it has been shown that cytochrome p-450 in the liver metabolizes sulfamethoxazole to its hydroxylamine metabolite. In this paper the N4-oxidation of sulfamethoxazole by activated monocytes and neutrophils (human and canine) to form sulfamethoxazole hydroxylamine and nitrosulfamethoxazole is reported. The presumed nitroso intermediate was not detected. Purified myeloperoxidase and prostaglandin H synthase were also capable of mediating the oxidation of sulfamethoxazole. The present studies suggest that myeloperoxidase is responsible for the observed oxidation by phagocytic cells. Oxidation by neutrophils may play a role in agranulocytosis, and oxidation by monocytes may facilitate antigen presentation. Extrahepatic bioactivation of sulfonamides by peroxidases in phagocytic cells and other tissues may be important in determining the range of adverse reactions to sulfonamides that occur.
PMID:2172779 Cribb AE et al; Mol Pharmacol 38 (5): 744-51 (1990)
Hypersensitivity syndromes are severe drug induced side effects with skin rashes, fever and/or multiorgan-system abnormalities which are not pharmacologically related. ... These reactions are considered to be immune-mediated but the precise mechanisms are not completely understood. Clinical features, which resemble and EBV infection, and some immunological studies suggest that T-cell mediated immunity is involved in the pathogenesis of this rare disease. In the literature, allopurinol and anticonvulsant hypersensitivity syndromes are clinically well characterized entities, while the definition of hypersensitivity syndrome elicited by other drugs is rather confusing. ... Two patients, one with sulfamethoxazole- and one with allopurinol-induced hypersensitivity syndrome /are presented/. In both cases a lymphocyte transformation test (LTT) was performed and /investigators/ analyzed the T-cell activation parameters CD25 and HLA-DR on CD4- and CD8- T-cells to demonstrate in vivo activation of T-cells during the active disease. Both patients show increased activation of T-cells with elevated levels of HLA-DR on CD8+ cells. The T-cell activation correlated with the clinical course. /The/ data supports an immunological pathogenesis for hypersensitivity syndromes and the concept that drug specific T-cells are involved in hypersensitivity syndromes.
PMID:9132934 Schnyder B et al; Schweiz Med Wochenschr 127 (9): 355-9 (1997)
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14982
Submission : 2000-07-25
Status : Inactive
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4291
Submission : 1981-09-25
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 6793
Submission : 1987-01-20
Status : Active
Type : II
Certificate Number : R1-CEP 1999-097 - Rev 03
Issue Date : 2015-12-08
Type : Chemical
Substance Number : 108
Status : Withdrawn by EDQM F...

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8211
Submission : 1989-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3466
Submission : 1979-02-19
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7177
Submission : 1987-10-02
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15950
Submission : 2002-04-23
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7350
Submission : 1988-02-25
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1998-049 - Rev 00
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2004-02-01
Type : Chemical
Substance Number : 108

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-332 - Rev 01
Status : Valid
Issue Date : 2017-07-11
Type : Chemical
Substance Number : 108

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1999-097 - Rev 03
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2015-12-08
Type : Chemical
Substance Number : 108

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1999-172 - Rev 02
Status : Valid
Issue Date : 2019-10-10
Type : Chemical
Substance Number : 108

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
86
PharmaCompass offers a list of Sulfamethoxazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sulfamethoxazole manufacturer or Sulfamethoxazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sulfamethoxazole manufacturer or Sulfamethoxazole supplier.
PharmaCompass also assists you with knowing the Sulfamethoxazole API Price utilized in the formulation of products. Sulfamethoxazole API Price is not always fixed or binding as the Sulfamethoxazole Price is obtained through a variety of data sources. The Sulfamethoxazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sulfamethoxazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sulfamethoxazole, including repackagers and relabelers. The FDA regulates Sulfamethoxazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sulfamethoxazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sulfamethoxazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sulfamethoxazole supplier is an individual or a company that provides Sulfamethoxazole active pharmaceutical ingredient (API) or Sulfamethoxazole finished formulations upon request. The Sulfamethoxazole suppliers may include Sulfamethoxazole API manufacturers, exporters, distributors and traders.
click here to find a list of Sulfamethoxazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sulfamethoxazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Sulfamethoxazole active pharmaceutical ingredient (API) in detail. Different forms of Sulfamethoxazole DMFs exist exist since differing nations have different regulations, such as Sulfamethoxazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sulfamethoxazole DMF submitted to regulatory agencies in the US is known as a USDMF. Sulfamethoxazole USDMF includes data on Sulfamethoxazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sulfamethoxazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sulfamethoxazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sulfamethoxazole Drug Master File in Japan (Sulfamethoxazole JDMF) empowers Sulfamethoxazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sulfamethoxazole JDMF during the approval evaluation for pharmaceutical products. At the time of Sulfamethoxazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sulfamethoxazole suppliers with JDMF on PharmaCompass.
A Sulfamethoxazole CEP of the European Pharmacopoeia monograph is often referred to as a Sulfamethoxazole Certificate of Suitability (COS). The purpose of a Sulfamethoxazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Sulfamethoxazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Sulfamethoxazole to their clients by showing that a Sulfamethoxazole CEP has been issued for it. The manufacturer submits a Sulfamethoxazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Sulfamethoxazole CEP holder for the record. Additionally, the data presented in the Sulfamethoxazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Sulfamethoxazole DMF.
A Sulfamethoxazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Sulfamethoxazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Sulfamethoxazole suppliers with CEP (COS) on PharmaCompass.
A Sulfamethoxazole written confirmation (Sulfamethoxazole WC) is an official document issued by a regulatory agency to a Sulfamethoxazole manufacturer, verifying that the manufacturing facility of a Sulfamethoxazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Sulfamethoxazole APIs or Sulfamethoxazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Sulfamethoxazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Sulfamethoxazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sulfamethoxazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sulfamethoxazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sulfamethoxazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sulfamethoxazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sulfamethoxazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sulfamethoxazole suppliers with NDC on PharmaCompass.
Sulfamethoxazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sulfamethoxazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sulfamethoxazole GMP manufacturer or Sulfamethoxazole GMP API supplier for your needs.
A Sulfamethoxazole CoA (Certificate of Analysis) is a formal document that attests to Sulfamethoxazole's compliance with Sulfamethoxazole specifications and serves as a tool for batch-level quality control.
Sulfamethoxazole CoA mostly includes findings from lab analyses of a specific batch. For each Sulfamethoxazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sulfamethoxazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Sulfamethoxazole EP), Sulfamethoxazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sulfamethoxazole USP).
