
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
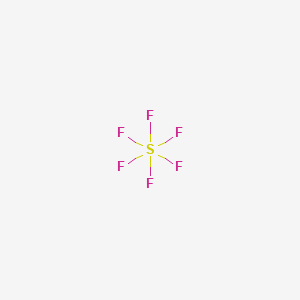

1. Hexafluoride, Sulfur
1. Sulphur Hexafluoride
2. 2551-62-4
3. Sulfur Fluoride
4. Sonovue
5. Elegas
6. Sulfur Fluoride (sf6)
7. Lumason
8. Sulfur(vi) Fluoride
9. Hexafluorure De Soufre
10. Hexafluoridosulfur
11. Sulfur Hexafluoride [usan]
12. Sulfur Fluoride (sf6), (oc-6-11)-
13. Sulfur(6+) Fluoride
14. Ws7lr3i1d6
15. Chebi:30496
16. Bri
17. Hexafluorosulfur
18. Sulfur Hexafluoride (usan)
19. Esaflon
20. Elagas
21. Sf6
22. Hsdb 825
23. Hexafluorure De Soufre [french]
24. Einecs 219-854-2
25. Sulfur Hexafluoride Lipid Microsphere
26. Un1080
27. Unii-ws7lr3i1d6
28. Sf6 Microbubbles
29. Sulfur Fluoride, (oc-6-11)-
30. Lumason (tn)
31. Sonovue (tn)
32. Hexafluoro-l6-sulfane
33. Hexafluoro-lambda6-sulfane
34. Ec 219-854-2
35. R 7146
36. Hexafluoro-$l^{6}-sulfane
37. Chembl1796998
38. Dtxsid8029656
39. Sulfur Hexafluoride [mi]
40. [sf6]
41. Sulfur Hexafluoride [hsdb]
42. Sulfur Hexafluoride, >=99.75%
43. Amy37163
44. Hexakis(fluoranyl)-$l^{6}-sulfane
45. Sulfur Hexafluoride [mart.]
46. Mfcd00011447
47. Sulfur Hexafluoride [who-dd]
48. Zinc245224194
49. Db11104
50. Oc-6-11
51. Sulphur Hexafluoride [ema Epar]
52. Un 1080
53. Sonovue (for The Microbubble Formulation)
54. Stabilized Sulfur Hexafluoride Microbubbles
55. Sulfur Hexafluoride, Phospholipid Microspheres
56. D05962
57. A817889
58. Q279055
59. Sulfur Hexafluoride [un1080] [nonflammable Gas]
60. Sulfur Hexafluoride Lipid-type A Microspheres [orange Book]
| Molecular Weight | 146.06 g/mol |
|---|---|
| Molecular Formula | F6S |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 145.96249015 g/mol |
| Monoisotopic Mass | 145.96249015 g/mol |
| Topological Polar Surface Area | 1 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lumason |
| Active Ingredient | Sulfur hexafluoride lipid-type a microspheres |
| Dosage Form | For suspension |
| Route | Intravenous |
| Strength | 60.7mg/25mg |
| Market Status | Prescription |
| Company | Bracco |
| 2 of 2 | |
|---|---|
| Drug Name | Lumason |
| Active Ingredient | Sulfur hexafluoride lipid-type a microspheres |
| Dosage Form | For suspension |
| Route | Intravenous |
| Strength | 60.7mg/25mg |
| Market Status | Prescription |
| Company | Bracco |
Lumason is indicated for use in patients with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
An inert gas used mainly as a test gas in respiratory physiology. Other uses include its injection in vitreoretinal surgery to restore the vitreous chamber ...
National Library of Medicine - Medical Subject Headings (2007)
The intraocular injection of sulfur hexafluoride appears to be useful contribution to the surgical treatment of superior bullous hemi-retinal detachment, allowing effective and durable internal tamponade, while avoiding prolonged bedrest.
J Fr Ophtalmol 6 (11): 889-93 (1983)
In this randomized clinical trial, 18 patients received treatment with silicone oil and 16 patients received SF6. The primary outcome was defined as successful anatomic attachment of the retina. Secondary outcomes included the time to retinal detachment, visual acuity, anatomic macular attachment, and any complications of surgery. RESULTS: The odds of successful reattachment with silicone oil were 50% greater than they were with SF6, but this difference was not statistically significant. There were no differences between the two groups in any of the secondary outcomes. ... This study did not have enough statistical power to detect a small but clinically important difference between the two treatment groups ...
PMID:9387180 Hammer M et al; Ophthalmic Surg Lasers 28 (11): 926-31 (1997)
For more Therapeutic Uses (Complete) data for SULFUR HEXAFLUORIDE (11 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: SERIOUS CARDIOPULMONARY REACTIONS Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration. Assess all patients for the presence of any condition that precludes administration. Always have resuscitation equipment and trained personnel readily available.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
Serious cardiopulmonary reactions, including fatalities have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including Lumason. These reactions typically occurred within 30 minutes of administration. The risk for these reactions may be increased among patients with unstable cardiopulmonary conditions (acute myocardial infarction, acute coronary artery syndromes, worsening or unstable congestive heart failure, or serious ventricular arrhythmias). Always have cardiopulmonary resuscitation personnel and equipment readily available prior to Lumason administration and monitor all patients for acute reactions. The reported reactions that may follow the administration of ultrasound contrast agents include: fatal cardiac or respiratory arrest, shock, syncope, symptomatic arrhythmias (atrial fibrillation, tachycardia, bradycardia, supraventricular tachycardia, ventricular fibrillation, and ventricular tachycardia), hypertension, hypotension, dyspnea, hypoxia, chest pain, respiratory distress, stridor, wheezing, loss of consciousness, and convulsions.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
Lumason is contraindicated in patients with: known or suspected right-to-left, bi-directional, or transient right-to-left cardiac shunts history of hypersensitivity reactions to sulfur hexafluoride lipid microsphere components or to any of the inactive ingredients in Lumason Do not administer by intra-arterial injection.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
In patients with right-to-left, bi-directional, or transient right-to-left cardiac shunts, some intravenously injected sulfur hexafluoride lipid containing microspheres may bypass filtering by the lung and directly enter the arterial circulation. Occlusion of the microcirculation by these microspheres may result in tissue ischemia. Lumason is only for intravenous administration; do not administer Lumason by intra-arterial injection.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
For more Drug Warnings (Complete) data for SULFUR HEXAFLUORIDE (19 total), please visit the HSDB record page.
Echocardiography: Sulfur hexafluoride is indicated for use in adult patients with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricularendocardial border. Ultrasonography of the Liver: Sulfur hexafluoride is indicated for use with ultrasound of the liver in adult and pediatric patients to characterize focal liver lesions.
This medicinal product is for diagnostic use only.
SonoVue is for use with ultrasound imaging to enhance the echogenicity of the blood, or of fluids in the urinary tract which results in an improved signal to noise ratio.
SonoVue should only be used in patients where study without contrast enhancement is inconclusive.
Echocardiography
SonoVue is a transpulmonary echocardiographic contrast agent for use in adult patients with suspected or established cardiovascular disease to provide opacification of cardiac chambers and enhance left ventricular endocardial border delineation.
Doppler of macrovasculature
SonoVue increases the accuracy in detection or exclusion of abnormalities in cerebral arteries and extracranial carotid or peripheral arteries in adult patients by improving the Doppler signal to noise ratio.
SonoVue increases the quality of the Doppler flow image and the duration of clinically useful signal enhancement in portal vein assessment in adult patients.
Doppler of microvasculature
SonoVue improves display of the vascularity of liver and breast lesions during Doppler sonography in adult patients leading to more specific lesion characterisation.
Ultrasonography of excretory urinary tract
SonoVue is indicated for use in ultrasonography of the excretory tract in paediatric patients from newborn to 18 years to detect vesicoureteral reflux. For the limitation in the interpretation of a negative urosonography.
Sulfur hexafluoride provides useful echocardiographic signal intensity for two minutes after the injection. Sulfur hexafluoride microspheres are destroyed and contrast enhancement decreases as the mechanical index increases (values of 0.8 or less are recommended). For ultrasonography of the liver, Sulfur hexafluoride provides dynamic patterns of differential signal intensity enhancement between focal liver lesions and liver parenchyma during the arterial, portal venous, and late phase of signal intensity enhancement of the microvasculature.
V08DA04
V08DA05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V08 - Contrast media
V08D - Ultrasound contrast media
V08DA - Ultrasound contrast media
V08DA05 - Sulfur hexafluoride, phospholipid microspheres
Absorption
The pharmacokinetic of the SF6 gas component of Lumason was evaluated in 12 healthy adult subjects (7 men and 5 women). After intravenous bolus injections of 0.03 mL/kg and 0.3 mL/kg of Lumason, corresponding to approximately 1 and 10 times the recommended doses, concentrations of SF6 in blood peaked within 1 to 2 minutes for both doses.
Route of Elimination
The SF6 component of Lumason is eliminated via the lungs.
Volume of Distribution
In a study of healthy subjects, the mean values for the apparent steady-state volume of distribution of SF6 were 341 L and 710 L for Lumason doses of 0.03 mL/kg and 0.3 mL/kg, respectively. Preferential distribution to the lung is likely responsible for these values.
SonoVue, is a new echo contrast agent based on stabilized sulfur hexafluoride (SF6) microbubbles ... The blood kinetics and pulmonary elimination of SF6 after intravenous bolus injection of two dosage levels (0.03 and 0.3 mL/kg) of SonoVue were evaluated in 12 healthy subjects (7 men, 5 women). In addition, safety and tolerability were evaluated by monitoring vital signs, adverse effects, discomfort, and physical examination and laboratory parameters associated with the SonoVue injection. The blood kinetics of SF6 was not dose dependent. SF6 was rapidly removed from the blood by the pulmonary route, with 40% to 50% of the injected dose eliminated within the first minute after administration and 80% to 90% eliminated by 11 minutes after administration; the elimination was similar in men and women and independent of dose. Both dosages were well tolerated. No adverse effects were observed immediately or during the 24-hour follow-up period. ... The route of SF6 elimination was by means of the lungs in the expired air. ...
PMID:10639039 Morel DR et al; Invest Radiol 35 (1): 80-5 (2000)
Sonovue (trade mark) is a new echo contrast agent made of microbubbles stabilized by phospholipids and containing sulphur hexafluoride (SF6), an innocuous gas. ... With regard to the gas contained in the bubbles, its pharmacokinetics have been assessed during a study in human volunteers. Following intravenous administration of 0.3 mL/kg of SonoVue (trade mark) (i.e., approximately ten times the imaging dose), the blood level curve showed a distribution half-life of about 1 minute and an elimination half-life of about 6 minutes. More than 80% of the administered gas is exhaled via the lungs after 11 minutes. ...
PMID:10639039 Schneider M; Echocardiography 16 (7, Pt 2): 743-746 (1999 Oct)
The pharmacokinetic of the SF6 gas component of Lumason was evaluated in 12 healthy adult subjects (7 men and 5 women). After intravenous bolus injections of 0.03 mL/kg and 0.3 mL/kg of Lumason, corresponding to approximately 1 and 10 times the recommended doses, concentrations of SF6 in blood peaked within 1 to 2 minutes for both doses. The terminal half-life of SF6 in blood was approximately 10 minutes for the 0.3 mL/kg dose. (At the 0.03 mL/kg dose, terminal half-life could not be estimated.) The area-under-the-curve of SF6 was dose-proportional over the dose range studied.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
In a study of healthy subjects, the mean values for the apparent steady-state volume of distribution of SF6 were 341 L and 710 L for Lumason doses of 0.03 mL/kg and 0.3 mL/kg, respectively. Preferential distribution to the lung is likely responsible for these values.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
For more Absorption, Distribution and Excretion (Complete) data for SULFUR HEXAFLUORIDE (6 total), please visit the HSDB record page.
SF6 undergoes little or no biotransformation; 88% of an administered dose is recovered unchanged in expired air.
SF6 undergoes little or no biotransformation; 88% of an administered dose is recovered unchanged in expired air.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
Under brief and prolonged exposures, sulfur hexafluoride was biologically inert and did not metabolize.
PMID:7250729 Mel'nikova LV, Rozova TA; Gig Tr Prof Zabol 6: 48-9 (1981)
The terminal half-life of SF6 in blood was approximately 10 minutes for the 0.3 mL/kg dose. (At the 0.03 mL/kg dose, terminal half-life could not be estimated.)
The pharmacokinetic of the SF6 gas component of Lumason was evaluated in 12 healthy adult subjects (7 men and 5 women). After intravenous bolus injections of 0.03 mL/kg and 0.3 mL/kg of Lumason... The terminal half-life of SF6 in blood was approximately 10 minutes for the 0.3 mL/kg dose. (At the 0.03 mL/kg dose, terminal half-life could not be estimated.) The area-under-the-curve of SF6 was dose-proportional over the dose range studied.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
... Following intravenous administration of 0.3 mL/kg of SonoVue (trade mark) (i.e., approximately ten times the imaging dose), the blood level curve showed a distribution half-life of about 1 minute and an elimination half-life of about 6 minutes. ...
PMID:10639039 Schneider M; Echocardiography 16 (7, Pt 2): 743-746 (1999 Oct)
In a study of patients with pulmonary impairment, blood concentrations of SF6 peaked at 1 to 4 minutes following Lumason administration. The cumulative recovery of SF6 in expired air was 102 +/- 18% (mean +/- standard deviation), and the terminal half-life of SF6 in blood was similar to that measured in healthy subjects.
NIH; DailyMed. Current Medication Information for LUMASON- sulfur hexafluoride (Revised: October 2014). Available from, as of August 2, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c679424-c3f7-ed02-892f-20ca0d775089
Within the blood, the acoustic impedance of Lumason microspheres is lower than that of the surrounding non-aqueous tissue. Therefore, an ultrasound beam is reflected from the interface between the microspheres and the surrounding tissue. The reflected ultrasound signal provides a visual image that shows a contrast between the blood and the surrounding tissues.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
28
PharmaCompass offers a list of Sulfur Hexafluoride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sulfur Hexafluoride manufacturer or Sulfur Hexafluoride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sulfur Hexafluoride manufacturer or Sulfur Hexafluoride supplier.
PharmaCompass also assists you with knowing the Sulfur Hexafluoride API Price utilized in the formulation of products. Sulfur Hexafluoride API Price is not always fixed or binding as the Sulfur Hexafluoride Price is obtained through a variety of data sources. The Sulfur Hexafluoride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A SULFUR manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of SULFUR, including repackagers and relabelers. The FDA regulates SULFUR manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. SULFUR API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A SULFUR supplier is an individual or a company that provides SULFUR active pharmaceutical ingredient (API) or SULFUR finished formulations upon request. The SULFUR suppliers may include SULFUR API manufacturers, exporters, distributors and traders.
click here to find a list of SULFUR suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A SULFUR DMF (Drug Master File) is a document detailing the whole manufacturing process of SULFUR active pharmaceutical ingredient (API) in detail. Different forms of SULFUR DMFs exist exist since differing nations have different regulations, such as SULFUR USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A SULFUR DMF submitted to regulatory agencies in the US is known as a USDMF. SULFUR USDMF includes data on SULFUR's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The SULFUR USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of SULFUR suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a SULFUR Drug Master File in Korea (SULFUR KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of SULFUR. The MFDS reviews the SULFUR KDMF as part of the drug registration process and uses the information provided in the SULFUR KDMF to evaluate the safety and efficacy of the drug.
After submitting a SULFUR KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their SULFUR API can apply through the Korea Drug Master File (KDMF).
click here to find a list of SULFUR suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing SULFUR as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for SULFUR API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture SULFUR as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain SULFUR and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a SULFUR NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of SULFUR suppliers with NDC on PharmaCompass.
SULFUR Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of SULFUR GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right SULFUR GMP manufacturer or SULFUR GMP API supplier for your needs.
A SULFUR CoA (Certificate of Analysis) is a formal document that attests to SULFUR's compliance with SULFUR specifications and serves as a tool for batch-level quality control.
SULFUR CoA mostly includes findings from lab analyses of a specific batch. For each SULFUR CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
SULFUR may be tested according to a variety of international standards, such as European Pharmacopoeia (SULFUR EP), SULFUR JP (Japanese Pharmacopeia) and the US Pharmacopoeia (SULFUR USP).
