



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
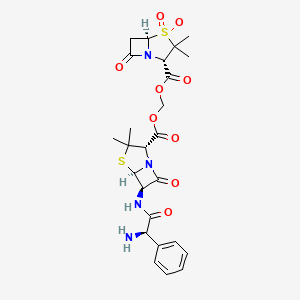

1. Ampicillin-sulbactam
2. Combisid
3. Cp 49,952
4. Cp 49952
5. Unasyn
1. 76497-13-7
2. Sultamicilina
3. Sultamicillinum
4. Sultamicillin Tosylate
5. Vd 1827
6. Cp-49,952
7. Chebi:51770
8. 65dt0ml581
9. 76497-13-7 (free)
10. Unacid
11. Vd-1827
12. Cp-49952
13. 4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid, 6-[[(2r)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-,[[[(2s,5r)-3,3-dimethyl-4,4-dioxido-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl]oxy]methylester, (2s,5r,6r)-
14. Hydroxymethyl (2s,5r,6r)-6-((r)-(2-amino-2-phenylacetamido))-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate, (2s,5r)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate (ester) S,s-dioxide
15. (((2s,5r,6r)-6-((r)-2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbonyl)oxy)methyl (2s,5r)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide
16. ({6beta-[(2r)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carbonyl}oxy)methyl 2,2-dimethylpenam-3alpha-carboxylate 1,1-dioxide
17. [(2,2-dimethyl-1,1-dioxidopenam-3alpha-carbonyl)oxy]methyl 6beta-[(2r)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylate
18. Sultamicilina [spanish]
19. Sultamicillinum [latin]
20. Cp 49952
21. Unii-65dt0ml581
22. Sultamicillin [usan:inn:ban]
23. Sultamicillin [mi]
24. Sultamicillin [inn]
25. Sultamicillin (usan/inn)
26. Sultamicillin [usan]
27. Schembl34392
28. Sultamicillin [mart.]
29. Sultamicillin [who-dd]
30. Chembl506110
31. Dtxsid501010077
32. Hy-n7115
33. Sultamicillin [ep Monograph]
34. Sultamicillin For Peak Identification
35. S5411
36. Zinc42834847
37. Akos015963376
38. Ccg-270182
39. Db12127
40. (2s,5r)-3,3-dimethyl-4,4,7-trioxo-4-thia-1-azabicyclo(3.2.0)heptan-2-carbonyloxymethyl (2s,5r,6r)-6-((r)-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptan-2-carboxylat
41. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((aminophenylacetyl)amino)-3,3-dimethyl-7-oxo-, (((3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)hept-2-yl)carbonyl)oxy)methyl Ester, S,s-dioxide, (2s-(2alpha(2r*,5s*),5alpha,6beta(s*)))-
42. As-14087
43. Cs-0082687
44. D05972
45. 497s137
46. 6'-(2-amino-2-phenylacetamido)penicillanoyloxymethyl Penicillanate 1,1-dioxide
47. [(2s,5r)-3,3-dimethyl-4,4,7-trioxo-4lambda6-thia-1-azabicyclo[3.2.0]heptane-2-carbonyl]oxymethyl (2s,5r,6r)-6-[[(2r)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
48. 1,1-dioxopenicillanoyloxymethyl 6-(d-.alpha.-amino-.alpha.-phenylacetamido)penicillanate
| Molecular Weight | 594.7 g/mol |
|---|---|
| Molecular Formula | C25H30N4O9S2 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 594.14542089 g/mol |
| Monoisotopic Mass | 594.14542089 g/mol |
| Topological Polar Surface Area | 216 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1240 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CR - Combinations of penicillins, incl. beta-lactamase inhibitors
J01CR04 - Sultamicillin


