
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
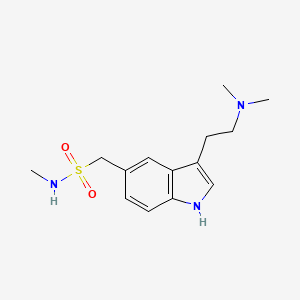

1. 3-(2-(dimethylamino)ethyl)-n-methyl-1h-indole-5-methanesulfonamide
2. Gr 43175
3. Gr-43175
4. Gr43175
5. Imigran
6. Succinate, Sumatriptan
7. Sumatriptan Succinate
1. 103628-46-2
2. Sumatran
3. Sumax
4. Imigran
5. Imitrex
6. Sumatriptanum
7. Gr-43175
8. 3-(2-(dimethylamino)ethyl)-n-methyl-1h-indole-5-methanesulfonamide
9. Gr 43175
10. 1-{3-[2-(dimethylamino)ethyl]-1h-indol-5-yl}-n-methylmethanesulfonamide
11. 3-[2-(dimethylamino)ethyl]-n-methylindole-5-methanesulfonamide
12. N02cc01
13. 1-[3-(2-dimethylaminoethyl)-1h-indol-5-yl]-n-methyl-methanesulfonamide
14. Imitrex (tn)
15. Chembl128
16. 1-[3-[2-(dimethylamino)ethyl]-1h-indol-5-yl]-n-methylmethanesulfonamide
17. 8r78f6l9vo
18. Chebi:10650
19. 3-[2-(dimethylamino)ethyl]-n-methyl-1h-indole-5-methanesulfonamide
20. (3-[2-(dimethylamino)ethyl]-1h-indol-5-yl)-n-methylmethanesulfonamide
21. 1-[3-(2-dimethylaminoethyl)-1h-indol-5-yl]-n-methylmethanesulfonamide
22. 1h-indole-5-methanesulfonamide, 3-(2-(dimethylamino)ethyl)-n-methyl-
23. 1h-indole-5-methanesulfonamide, 3-[2-(dimethylamino)ethyl]-n-methyl-
24. Ncgc00095838-01
25. Sumatriptanum [inn-latin]
26. Dsstox_cid_3628
27. 1-(3-(2-(dimethylamino)ethyl)-1h-indol-5-yl)-n-methylmethanesulfonamide
28. Dsstox_rid_77118
29. Dsstox_gsid_23628
30. Zecuity
31. Gr 43175x
32. Smr000596517
33. Imigran (tn)
34. Cas-103628-46-2
35. Brn 6930870
36. Unii-8r78f6l9vo
37. Sumatriptan (jan/usp/inn)
38. Sumatriptan [usp:inn:ban]
39. Avp825
40. Imigran Recovery
41. {3-[2-(dimethylamino)ethyl]-1h-indol-5-yl}-n-methylmethanesulfonamide
42. Hsdb 7742
43. Avp 825
44. Avp-825
45. Sumatriptansuccinate
46. Sumatriptan- Bio-x
47. Tosymra
48. Tosymra (nasal Spray)
49. Sumatriptan [mi]
50. Sumatriptan [inn]
51. Sumatriptan [jan]
52. Sumatriptan [hsdb]
53. Gtpl54
54. Sumatriptan [vandf]
55. Schembl1482
56. Sumatriptan [mart.]
57. Bspbio_002304
58. Sumatriptan [usp-rs]
59. Sumatriptan [who-dd]
60. Mls001195659
61. Mls001304742
62. Bidd:gt0248
63. Spectrum1505372
64. Dfn-11
65. Dtxsid4023628
66. Hy-b0121b
67. Zinc14360
68. Sumatriptan [orange Book]
69. Hms2231b21
70. Hms3369a05
71. Sumatriptan [usp Monograph]
72. Amy17769
73. Bcp02237
74. Tox21_111528
75. Bdbm50005835
76. Stl451003
77. Akos015894924
78. Tox21_111528_1
79. Ccg-230331
80. Db00669
81. Dfn-11 (sumatriptan Injection)
82. Ncgc00095838-02
83. Ncgc00095838-03
84. Ncgc00095838-04
85. Bs164424
86. Sbi-0206837.p001
87. Cs-0009565
88. Ft-0631027
89. Ft-0674779
90. C07319
91. C90357
92. D00451
93. Ab00698285-12
94. Ab00698285-13
95. Ab00698285_14
96. Ab00698285_15
97. 628s462
98. L000584
99. Q416978
100. J-001014
101. Brd-k50938287-001-01-7
102. 3-[2-(dimethylamino)ethyl]-n-methyl-1h-indole-5-methanesulphonamide
103. (3-[2-(dimethylamino)ethyl]-1h-indol-5-yl)-n-methylmethanesulfonamide #
104. 3-(2-(dimethylamino)ethyl)-n-methy-1h-indole-5-methanesulfonamide
105. 3-[2-(dimethylamino)ethyl]-n-methyl-1h-indole -5-methanesulphonamide
| Molecular Weight | 295.40 g/mol |
|---|---|
| Molecular Formula | C14H21N3O2S |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 295.13544809 g/mol |
| Monoisotopic Mass | 295.13544809 g/mol |
| Topological Polar Surface Area | 73.6 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 405 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Alsuma |
| PubMed Health | Sumatriptan |
| Drug Classes | Antimigraine |
| Drug Label | Sumatriptan is a selective 5-hydroxy-tryptamine receptor subtype 1 (5-HT1) agonist. Sumatriptan delivered as the succinate salt is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has th... |
| Active Ingredient | Sumatriptan succinate |
| Dosage Form | Injectable |
| Route | Subcutaneous |
| Strength | eq 6mg base/0.5ml (eq 12mg base/ml) |
| Market Status | Prescription |
| Company | Meridian Medcl |
| 2 of 6 | |
|---|---|
| Drug Name | Imitrex |
| PubMed Health | Sumatriptan |
| Drug Classes | Antimigraine |
| Drug Label | IMITREX Tablets contain sumatriptan (as the succinate), a selective 5-hydroxytryptamine1 receptor subtype agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and... |
| Active Ingredient | Sumatriptan; Sumatriptan succinate |
| Dosage Form | Tablet; Spray; Injectable |
| Route | Oral; Nasal; Subcutaneous |
| Strength | eq 100mg base; 20mg/spray; eq 50mg base; eq 6mg base/0.5ml (eq 12mg base/ml); eq 25mg base; 5mg/spray |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 6 | |
|---|---|
| Drug Name | Zecuity |
| PubMed Health | Sumatriptan |
| Drug Classes | Antimigraine |
| Drug Label | ZECUITY (sumatriptan iontophoretic transdermal system) is a disposable, single use system designed to deliver sumatriptan through the skin using iontophoresis. Iontophoresis is a non-invasive method of delivering a drug through the skin using a low e... |
| Active Ingredient | Sumatriptan succinate |
| Dosage Form | System |
| Route | Iontophoresis |
| Strength | eq 6.5mg base/4hr |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
| 4 of 6 | |
|---|---|
| Drug Name | Zecuity |
| PubMed Health | Sumatriptan |
| Drug Classes | Antimigraine |
| Drug Label | ZECUITY (sumatriptan iontophoretic transdermal system) is a disposable, single use system designed to deliver sumatriptan through the skin using iontophoresis. Iontophoresis is a non-invasive method of delivering a drug through the skin using a low e... |
| Active Ingredient | Sumatriptan succinate |
| Dosage Form | System |
| Route | Iontophoresis |
| Strength | eq 6.5mg base/4hr |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
| 5 of 6 | |
|---|---|
| Drug Name | Alsuma |
| PubMed Health | Sumatriptan |
| Drug Classes | Antimigraine |
| Drug Label | Sumatriptan is a selective 5-hydroxy-tryptamine receptor subtype 1 (5-HT1) agonist. Sumatriptan delivered as the succinate salt is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has th... |
| Active Ingredient | Sumatriptan succinate |
| Dosage Form | Injectable |
| Route | Subcutaneous |
| Strength | eq 6mg base/0.5ml (eq 12mg base/ml) |
| Market Status | Prescription |
| Company | Meridian Medcl |
| 6 of 6 | |
|---|---|
| Drug Name | Imitrex |
| PubMed Health | Sumatriptan |
| Drug Classes | Antimigraine |
| Drug Label | IMITREX Tablets contain sumatriptan (as the succinate), a selective 5-hydroxytryptamine1 receptor subtype agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and... |
| Active Ingredient | Sumatriptan; Sumatriptan succinate |
| Dosage Form | Tablet; Spray; Injectable |
| Route | Oral; Nasal; Subcutaneous |
| Strength | eq 100mg base; 20mg/spray; eq 50mg base; eq 6mg base/0.5ml (eq 12mg base/ml); eq 25mg base; 5mg/spray |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Serotonin Agonists; Vasoconstrictor Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Sumatriptan tablets are indicated for the acute treatment of migraine attacks with or without aura in adults. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Imitrex (sumatriptan succinate) tablets (January 2008). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6576
Sumatriptan tablets are NOT intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine. Safety and effectiveness of sumatriptan tablets have not been established for cluster headache, which is present in an older, predominantly male population. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Imitrex (sumatriptan succinate) tablets (January 2008). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6576
/EXPTL Ther:/ To determine the impact of sumatriptan prophylaxis on acute mountain sickness (AMS) and altitude headache development within 24 hours of ascent, we designed a double-blind, randomized, clinical trial. A prospective, double-blind, randomized, placebo-controlled trial was conducted in Tochal Mountain Hotel at an altitude of 3,500 meters above sea level during October 2006 to November 2006. A total of 102 Iranian adults were assigned to receive either sumatriptan succinate (50mg) or placebo within 1 hour of ascent. AMS incidence was measured by Lake Louise AMS score > or = 3 with headache and one other symptom. Secondary outcome measures included severity of syndrome (Lake Louise scores > or = 5), incidence of headache, and severity of headache. Based on intention-to-treat analysis, AMS was more prevalent in placebo group (n = 23 [45.1%]) than sumatriptan group (n = 12 [23.5%]; p = 0.02). Headache also had a greater rate for placebo users (placebo vs sumatriptan group: 29 [56.9%] vs 17 [33.3%]; p = 0.02). No association was detected between sumatriptan prophylaxis and AMS or altitude headache severity. Sumatriptan prophylaxis is effective to prevent AMS development. Furthermore, our findings confirm cerebral vasodilative and edematous mechanisms of AMS progression, whereas sumatriptan is a selective 5-hydroxytryptamine(1) receptor subtype agonist and a selective cerebral vasoconstrictor as a result
PMID:17557349 Jafarian S et al; Ann Neurol 62 (3): 273-7 (2007).
/EXPTL Ther:/ ... A patient with a 4-year history of cyclic vomiting was treated for an episode of nausea, vomiting, and abdominal pain. This patient had been hospitalized numerous times for cyclic vomiting over the previous 4 years, each hospitalization lasting from 3 to 11 days. Following a single subcutaneous injection of sumatriptan 6 mg, the patient ceased vomiting and was discharged 40 hours from the time of admission. The efficacy of sumatriptan in migraine headache appears to be mediated through its agonist activity at the serotonin1D receptor, resulting in constriction of dural blood vessels. According to published reports, therapeutic attempts at controlling cyclic vomiting often have included antimigraine therapies. Consistent with these reports, sumatriptan also appears effective in the treatment of cyclic vomiting. The pathogenesis of cyclic vomiting appears to share similarities with classic migraine, both of which may respond to sumatriptan therapy according to this report and previous work. Further study of the use of sumatriptan in the treatment of cyclic vomiting appears warranted.
PMID:8845562 Benson JM et al; Ann Pharmacother 29 (10): 997-9 (1995).
Most adverse effects associated with sumatriptan are well defined, transient, and mild to moderate in intensity, although serious cardiac events (coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, ventricular fibrillation) have been reported rarely in patients receiving the drug subcutaneously or orally. Adverse effects associated with the drug usually occur within 1 hour after subcutaneous or oral administration of sumatriptan and generally resolve within 10-30 minutes (subcutaneous) or 1 hour (oral). The incidence of adverse effects associated with sumatriptan generally remains unchanged or decreases with repeated use of the drug. However, the incidence of adverse effects appears to increase with higher than recommended doses of the drug. In addition, the overall incidence of adverse effects among patients receiving sumatriptan injection for the treatment of cluster headache is lower than that in patients being treated with the drug for migraine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2661
The most frequently reported adverse effects associated with subcutaneous sumatriptan succinate therapy are injection site reaction (eg, minor pain, edema, tingling at the site of injection, burning, transient erythema), tingling, dizziness or vertigo, and sensations of warmth or heat. Common adverse effects reported in patients receiving oral sumatriptan for the treatment of migraine or cluster headache include malaise or fatigue, nausea or vomiting, dizziness or vertigo, tingling, and nasal discomfort. Since some adverse effects noted with sumatriptan therapy (eg, nausea or gastric symptoms, tingling, photophobia, visual disturbances, headache, numbness, neck pain, drowsiness/sedation, asthenia, fatigue) also are symptoms associated with migraine attacks and/or the postdromal period, it may be difficult to distinguish the effects of underlying disease processes from drug-induced effects. The most frequently reported adverse effects associated with intranasal sumatriptan include disturbances of taste, nausea or vomiting, and disease of nasal cavity or sinuses. For adverse effects reported with sumatriptan therapy in the Cautions section, a causal relationship to the drug has not always been established. In addition, the incidence of adverse effects reported in clinical trials may not predict precisely the likelihood of encountering these effects under usual medical practice where patient characteristics and other factors differ from those prevailing in the trials.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2661
Pooled data from controlled studies indicate that the most frequently reported adverse effect associated with subcutaneous sumatriptan succinate therapy is injection site reaction, consisting of minor pain, edema, induration, swelling, contusions, subcutaneous bleeding, stinging or tingling at the site of injection, burning, and/or transient erythema. Lipoatrophy (depression in the skin) or lipohypertrophy (enlargement or thickening of tissue) has been reported in less than 0.1% of patients receiving the drug subcutaneously. Injection site reactions occurred in 58.7% of patients receiving the drug subcutaneously in controlled trials; this effect occurred with less frequency in patients using an auto-injector to administer the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2661
Nasal and/or throat irritation were reported in approximately 5% of patients receiving 5-, 10-, or 20-mg doses of intranasal sumatriptan on 1 or 2 occasions in controlled clinical studies. Transient irritative symptoms (eg, burning, numbness, paresthesia, discharge, pain or soreness) were reported to be severe in about 1% of patients receiving intranasal sumatriptan; these symptoms generally resolved in less than 2 hours. Limited examination of the nose and throat did not reveal clinically noticeable injury in these patients. In addition, an increased incidence of local irritation has not been observed in patients receiving intranasal sumatriptan repeatedly for up to 1 year. However, epithelial hyperplasia (with and without keratinization) and squamous metaplasia were observed in the larynx of rats receiving inhaled sumatriptan daily for 1 month at dosages as low as one-half the maximum daily human exposure (based on dose per surface area of nasal cavity). In addition, evidence of epithelial hyperplasia, focal squamous metaplasia, granulomata, bronchitis, and fibrosing alveolitis was observed in the respiratory and nasal mucosa in dogs administered various formulations of sumatriptan by intranasal instillation daily for up to 13 weeks, at exposure rates of 2-4 times the maximum daily human exposure (based on dose per surface area of nasal cavity). The changes observed in both species are not considered to be signs of preneoplastic or neoplastic transformation. Local effects on nasal and respiratory tissues after chronic, repeated intranasal administration of sumatriptan have not been studied in animals or humans.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2661
For more Drug Warnings (Complete) data for Sumatriptan (42 total), please visit the HSDB record page.
A combination sumatriptan and [naproxen] tablet is indicated for the treatment of migraines with or without auras in patients 12 years of age and older. Sumatriptan nasal powder, nasal spray, subcutaneous injection, and tablets are indicated to treat migraines with or without auras in adults. One of the subcutaneous formulations of sumatriptan is also indicated to treat cluster headaches in adults, while the other subcutaneous formulation is not.
Sumatriptan constricts cranial blood vessels and prevents the release of vasoactive peptides. The dose of sumatriptan varies widely by route of administration and in most cases, no more than 2 doses should be given daily. Medication overuse headaches may occur in patients who use sumatriptan frequently.
Serotonin 5-HT1 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT1 RECEPTORS. Included under this heading are agonists for one or more of the specific 5-HT1 receptor subtypes. (See all compounds classified as Serotonin 5-HT1 Receptor Agonists.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
N02CC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N02 - Analgesics
N02C - Antimigraine preparations
N02CC - Selective serotonin (5ht1) agonists
N02CC01 - Sumatriptan
Absorption
A 6mg subcutaneous injection of sumatriptan reaches a Cmax of 69.5ng/mL (95% CI of 62.8-76.9ng/mL) with a Tmax of 0.17h (95% CI of 0.08-0.33h), an AUC of 9.0h\*ng/mL (95% CI of 7.5-10.9h\*ng/mL), and a bioavailability of 100%. A 25mg oral dose of sumatriptan reaches a Cmax of 16.5ng/mL (95% CI of 13.5-20.1ng/mL) with a Tmax of 1.50h (95% CI of 0.50-2.00h), an AUC of 8.7h\*ng/mL (95% CI of 6.1-12.5h\*ng/mL), and a bioavailability of 14.3% (95% CI of 11.4-17.9%). A 20mg intranasal dose of sumatriptan reaches a Cmax of 12.9ng/mL (95% CI of 10.5-15.9ng/mL) with a Tmax of 1.50h (95% CI of 0.25-3.00h), an AUC of 7.4h\*ng/mL (95% CI of 5.0-10.8h\*ng/mL), and a bioavailability of 15.8% (95% CI of 12.6-19.8%). A 25mg rectal dose of sumatriptan reaches a Cmax of 22.9ng/mL (95% CI of 18.4-28.6ng/mL) with a Tmax of 1.00h (95% CI of 0.75-3.00h), an AUC of 14.6h\*ng/mL (95% CI of 11.3-18.8h\*ng/mL), and a bioavailability of 19.2% (95% CI of 15.3-24.1%).
Route of Elimination
224% is excreted in the urine as unchanged sumatriptan and 387% in urine as indole acetic acid approximately 40% is excreted in the feces.
Volume of Distribution
Sumatriptan has a volume of distribution of 508L for a 6mg subcutaneous dose, or 2.7L/kg.
Clearance
Subcutaneous sumatriptan has a clearance of 0.22L/min (95% CI of 0.19-0.25L/min). Oral sumatriptan has a clearance of 0.17L/min (95% CI of 0.14-0.21L/min). Rectal sumatriptan has a clearance of 0.17L/min (95% CI of 0.14-0.21L/min). Intrsnasal sumatriptan has a clearance of 0.21L/min (95% CI of 0.18-0.25L/min). Total plasma clearance of sumatriptan is approximately 1200mL/min.
Sumatriptan is rapidly absorbed following subcutaneous or oral administration; oral absorption appears to occur in the small intestine. The drug also is absorbed rapidly following intranasal administration. The bioavailability of sumatriptan given subcutaneously is almost complete, averaging about 97% of that obtained with iv administration of the drug. The bioavailability of sumatriptan following oral or intranasal administration averages only about 15 or 17%, respectively, principally because of presystemic metabolism of the drug and in part because of incomplete absorption. The area under the plasma concentration-time curve (AUC) and peak serum concentration of sumatriptan increase linearly with single subcutaneous doses of 1-16 mg. The extent of sumatriptan absorption (AUC) also is dose-proportional following single oral doses of 25-200 mg; however, peak plasma concentrations after a 100-mg oral dose of sumatriptan are approximately 25% less than those predicted from a 25-mg oral dose.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2665
Interindividual variability in the absorption of sumatriptan after oral administration results in multiple peaks in plasma concentration, possibly because of differences in the rates of gastric emptying, small-bowel transit, and/or presystemic metabolism; however, 75-80% of the final peak plasma concentration is reached within 45 minutes after dosing. Administration of higher than recommended single oral doses of sumatriptan (ie, 200-400 mg) is associated with a decrease in the rate of absorption.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
Oral absorption of the drug does not appear to be affected appreciably by gastric stasis that may occur during a migraine attack; however, the time to peak concentration is prolonged by about 30 minutes. The pharmacokinetics of sumatriptan following subcutaneous injection reportedly are similar during migraine attacks and pain-free periods. Absorption of subcutaneous sumatriptan is not affected by race or gender.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
A food effect study involving administration of sumatriptan tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that the Cmax and AUC were increased by 15% and 12%, respectively, when administered in the fed state.
US Natl Inst Health; DailyMed. Current Medication Information for Imitrex (sumatriptan succinate) tablets (January 2008). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6576
For more Absorption, Distribution and Excretion (Complete) data for Sumatriptan (17 total), please visit the HSDB record page.
Sumatriptan is predominantly metabolized by monoamine oxidase A. The main metabolites are the inactive indole acetic acid and indole acetic acid glucuronide.
The principal metabolite of sumatriptan is its inactive indole acetic acid analog, which is formed by oxidative N-deamination of the N-dimethyl side chain. The indole acetic acid metabolite of sumatriptan achieves plasma concentrations 6-7 times higher than those of sumatriptan but has a half-life similar to that of the parent compound, suggesting that clearance of this metabolite is formation-rate limited. Other minor metabolites of sumatriptan, an ester glucuronide of the indole acetic acid derivative and an indole ethyl alcohol derivative, also have been identified.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
Metabolism is the principal clearance process for sumatriptan. Sumatriptan is metabolized in the liver and possibly in the GI tract and is eliminated in urine and feces. In vitro studies suggest that sumatriptan is metabolized by monoamine oxidase (MAO), principally the A isoenzyme (MAO-A); inhibitors of this enzyme may increase systemic exposure to sumatriptan.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
Subcutaneous sumatriptan has a half life of 1.9h (95% CI of 1.7-2.0h). Oral sumatriptan has a half life of 1.7h (95% CI of 1.4-1.9h). Rectal sumatriptan has a half life of 1.8h (95% CI of 1.6-2.2h). Intrsnasal sumatriptan has a half life of 1.8h (95% CI of 1.7-2.0h).
Following single subcutaneous or oral doses of sumatriptan in healthy individuals, the terminal elimination half-life of the drug is 1.5-2.6 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
Following single-dose oral administration of large doses of sumatriptan or repeated administration of smaller doses, a second terminal elimination phase has been observed but not characterized. The prolonged elimination half-life with multiple dosing or administration of large single doses may indicate enterohepatic recycling or prolonged oral absorption and does not appear to affect substantially the disposition of the drug. Most of a dose of sumatriptan is excreted within 10-24 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
Following intranasal administration of sumatriptan, the elimination half-life reportedly is about 2 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2666
Sumatriptan is an agonist of 5-HT1B and 5-HT1D. This agonism leads to constriction of cranial blood vessels and inhibits the release of pro-inflammatory neuropeptides. Sumatriptan decreases carotid arterial blood flow, but increases blood flow velocity in the internal carotid artery and middle cerebral artery.[A179734 Agonism of the 5-HT1B and 5-HT1D receptors also inhibits sensory neurons, preventing the release of vasoactive peptides.[A179734 Sumatriptan does not cross the blood brain barrier.
Sumatriptan and other currently available drugs that are effective for acute migraine, including dihydroergotamine and ergotamine, have binding affinity for serotonin type 1 (5-HT1) receptors, particularly the 5-HT1D (also called 5-HT1Dalpha) and 5-HT1B (also called 5-HT1Dbeta) subtypes located on trigeminal sensory neurons innervating dural blood vessels. The 5-HT1B and 5-HT1D receptors function as autoreceptors, activation of which leads to inhibition of firing of serotonin neurons and a reduction in the synthesis and release of serotonin. Upon binding to these 5-HT1 receptor subtypes, sumatriptan inhibits adenylate cyclase activity via regulatory G proteins, increases intracellular calcium, and affects other intracellular events that lead to vasoconstriction and inhibition of sensory nociceptive (trigeminal) nerve firing and vasoactive neuropeptide release. Sumatriptan has the highest affinity for the 5-HT1D receptor, the most common serotonin receptor subtype in the brain, and a 2- to 17-fold lower affinity for 5-HT1A receptors; agonist activity at 5-HT1A and other serotonin receptors may be responsible for some of the adverse effects noted with administration of serotonin or serotonergic antimigraine drugs (eg, ergotamine, dihydroergotamine). Sumatriptan has essentially no affinity for (based on standard radioligand binding assays) nor pharmacologic activity at other serotonin receptors (eg, 5-HT2, 5-HT3) or at receptors of the dopamine1, dopamine2, muscarinic, histamine, benzodiazepine, or alpha1-, alpha2-, or beta-adrenergic type.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2664
Sumatriptan is a selective agonist of vascular serotonin (5-hydroxytryptamine; 5-HT) type 1-like receptors, probably the 5-HT1D and 5-HT1B subtypes. The mechanisms involved in the pathogenesis of migraine and cluster headache are not clearly understood; consequently, the precise mechanism of action of sumatriptan in the management of these disorders has not been established. However, current data suggest that sumatriptan may ameliorate migraine and cluster headache through selective constriction of certain large cranial blood vessels and/or inhibition of neurogenic inflammatory processes in the CNS. While some features of migraine clearly reflect effects on cerebral blood vessels, neurogenic mechanisms involving activation of the trigeminovascular system also have been implicated; current evidence suggests that both mechanisms may be involved.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2665
The vascular 5-HT1 receptor subtype that sumatriptan activates is present on cranial arteries in both dog and primate, on the human basilar artery, and in the vasculature of human dura mater and mediates vasoconstriction. This action in humans correlates with the relief of migraine headache. In addition to causing vasoconstriction, experimental data from animal studies show that sumatriptan also activates 5-HT1 receptors on peripheral terminals of the trigeminal nerve innervating cranial blood vessels. Such an action may also contribute to the antimigrainous effect of sumatriptan in humans.
US Natl Inst Health; DailyMed. Current Medication Information for Imitrex (sumatriptan succinate) tablets (January 2008). Available from, as of June 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6576
Sumatriptan selectively reduces carotid arterial blood flow and/or constricts carotid arteriovenous anastomoses in anesthetized animals without appreciable effects on arterial blood pressure or total peripheral resistance. The drug produces contraction of vascular smooth muscle in vitro in saphenous veins in dogs and humans, but such contractions are weaker than those produced by serotonin or ergot alkaloids (eg, methysergide).
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2665
For more Mechanism of Action (Complete) data for Sumatriptan (9 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
100
PharmaCompass offers a list of Sumatriptan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sumatriptan manufacturer or Sumatriptan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sumatriptan manufacturer or Sumatriptan supplier.
PharmaCompass also assists you with knowing the Sumatriptan API Price utilized in the formulation of products. Sumatriptan API Price is not always fixed or binding as the Sumatriptan Price is obtained through a variety of data sources. The Sumatriptan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sumatriptan Succinate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sumatriptan Succinate, including repackagers and relabelers. The FDA regulates Sumatriptan Succinate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sumatriptan Succinate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sumatriptan Succinate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sumatriptan Succinate supplier is an individual or a company that provides Sumatriptan Succinate active pharmaceutical ingredient (API) or Sumatriptan Succinate finished formulations upon request. The Sumatriptan Succinate suppliers may include Sumatriptan Succinate API manufacturers, exporters, distributors and traders.
click here to find a list of Sumatriptan Succinate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sumatriptan Succinate DMF (Drug Master File) is a document detailing the whole manufacturing process of Sumatriptan Succinate active pharmaceutical ingredient (API) in detail. Different forms of Sumatriptan Succinate DMFs exist exist since differing nations have different regulations, such as Sumatriptan Succinate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sumatriptan Succinate DMF submitted to regulatory agencies in the US is known as a USDMF. Sumatriptan Succinate USDMF includes data on Sumatriptan Succinate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sumatriptan Succinate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sumatriptan Succinate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sumatriptan Succinate Drug Master File in Japan (Sumatriptan Succinate JDMF) empowers Sumatriptan Succinate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sumatriptan Succinate JDMF during the approval evaluation for pharmaceutical products. At the time of Sumatriptan Succinate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sumatriptan Succinate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Sumatriptan Succinate Drug Master File in Korea (Sumatriptan Succinate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Sumatriptan Succinate. The MFDS reviews the Sumatriptan Succinate KDMF as part of the drug registration process and uses the information provided in the Sumatriptan Succinate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Sumatriptan Succinate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Sumatriptan Succinate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Sumatriptan Succinate suppliers with KDMF on PharmaCompass.
A Sumatriptan Succinate CEP of the European Pharmacopoeia monograph is often referred to as a Sumatriptan Succinate Certificate of Suitability (COS). The purpose of a Sumatriptan Succinate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Sumatriptan Succinate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Sumatriptan Succinate to their clients by showing that a Sumatriptan Succinate CEP has been issued for it. The manufacturer submits a Sumatriptan Succinate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Sumatriptan Succinate CEP holder for the record. Additionally, the data presented in the Sumatriptan Succinate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Sumatriptan Succinate DMF.
A Sumatriptan Succinate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Sumatriptan Succinate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Sumatriptan Succinate suppliers with CEP (COS) on PharmaCompass.
A Sumatriptan Succinate written confirmation (Sumatriptan Succinate WC) is an official document issued by a regulatory agency to a Sumatriptan Succinate manufacturer, verifying that the manufacturing facility of a Sumatriptan Succinate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Sumatriptan Succinate APIs or Sumatriptan Succinate finished pharmaceutical products to another nation, regulatory agencies frequently require a Sumatriptan Succinate WC (written confirmation) as part of the regulatory process.
click here to find a list of Sumatriptan Succinate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sumatriptan Succinate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sumatriptan Succinate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sumatriptan Succinate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sumatriptan Succinate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sumatriptan Succinate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sumatriptan Succinate suppliers with NDC on PharmaCompass.
Sumatriptan Succinate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sumatriptan Succinate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sumatriptan Succinate GMP manufacturer or Sumatriptan Succinate GMP API supplier for your needs.
A Sumatriptan Succinate CoA (Certificate of Analysis) is a formal document that attests to Sumatriptan Succinate's compliance with Sumatriptan Succinate specifications and serves as a tool for batch-level quality control.
Sumatriptan Succinate CoA mostly includes findings from lab analyses of a specific batch. For each Sumatriptan Succinate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sumatriptan Succinate may be tested according to a variety of international standards, such as European Pharmacopoeia (Sumatriptan Succinate EP), Sumatriptan Succinate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sumatriptan Succinate USP).
