
Synopsis
Synopsis
0
VMF
0
Weekly News Recap #Phispers
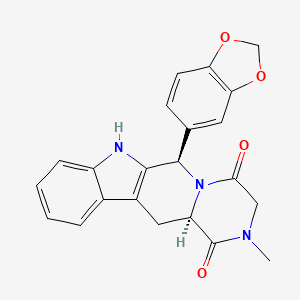

1. Cialis
2. Ic 351
3. Ic-351
4. Ic351
5. Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione, 6-(1,3- Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6r,12ar)-
1. Cialis
2. 171596-29-5
3. Ic351
4. Tadanafil
5. Adcirca
6. Ic 351
7. Icos 351
8. Ic-351
9. Gf 196960
10. (6r,12ar)-6-(benzo[d][1,3]dioxol-5-yl)-2-methyl-2,3,12,12a-tetrahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4(6h,7h)-dione
11. Tadalafil Mylan
12. Trans-tadalafil
13. Tadalafil(cialis)
14. (6r,12ar)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-(methylenedioxy)phenyl) Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione
15. (6r-trans)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione
16. Chembl779
17. Nsc-750236
18. Nsc-759172
19. 742sxx0ict
20. Gf-196960
21. Ly-450190
22. (6r,12ar)-6-(benzo[d][1,3]dioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
23. Chebi:71940
24. Tadalafil [usan]
25. Ly450190
26. Dsstox_cid_26786
27. Dsstox_rid_81904
28. Dsstox_gsid_46786
29. (6r,12ar)-6-(1,3-benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
30. 6-benzo[1,3]dioxol-5-yl-2-methyl-2,3,6,7,12,12a-hexahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
31. Tadalafil Lilly
32. Smr000466321
33. Cialis (tn)
34. Tildenafil
35. Cas-171596-29-5
36. Hsdb 7303
37. Unii-742sxx0ict
38. Tadalafila
39. Tadalafilo
40. Taldalafil
41. Tadalafil [usan:inn:ban]
42. Tadalafil Solution
43. 1xoz
44. Cialis, Tadalafil
45. Ncgc00168781-01
46. Tadalafil- Bio-x
47. (2r,8r)-2-(1,3-benzodioxol-5-yl)-6-methyl-3,6,17-triazatetracyclo[8.7.0.0^{3,8}.0^{11,16}]heptadeca-1(10),11,13,15-tetraene-4,7-dione
48. Adcirca (tn)
49. Tadalafil (cialis)
50. Zalutia (tn)
51. (6r,12ar)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione
52. Ks-1117
53. Tadalafil [inn]
54. Tadalafil [jan]
55. Tadalafil [mi]
56. Tadalafil; Cialis
57. Tadalafil [hsdb]
58. Tadalafil [vandf]
59. (-)-tadalafil (6r)
60. Tadalafil [mart.]
61. Tadalafil [usp-rs]
62. Tadalafil [who-dd]
63. Tadalafil (jan/usp/inn)
64. Schembl33333
65. Tadalafil [ema Epar]
66. Mls000759426
67. Mls001165782
68. Mls001195644
69. Mls001424132
70. Mls006010126
71. Tadalafil Specified Impurity A
72. Gtpl7299
73. Tadalafil [orange Book]
74. Dtxsid9046786
75. Tadalafil [ep Monograph]
76. Bdbm14777
77. Tadalafil (6r ,12as)- Lilly
78. Tadalafil [usp Monograph]
79. Entadfi Component Tadalafil
80. Hms2051n17
81. Hms2235l21
82. Hms3884g19
83. Pharmakon1600-01505639
84. Amy10321
85. Ex-a2644
86. Zinc3993855
87. Tox21_112642
88. Hy-90009a
89. Mfcd07771966
90. Nsc750236
91. Nsc759172
92. S1512
93. Tadalafil 1.0 Mg/ml In Acetonitrile
94. Tadalafil 100 Microg/ml In Methanol
95. Tadalafil Component Of Entadfi
96. Akos015892559
97. Tox21_112642_1
98. Ab42193
99. Ccg-100973
100. Cs-1414
101. Db00820
102. Nc00223
103. Nsc 750236
104. Nsc 759172
105. 1,3-benzodioxol-5-yl(methyl)[?]dione
106. Ncgc00263909-02
107. Tadalafil 100 Microg/ml In Acetonitrile
108. (6r,12ar)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
109. Bt164435
110. Gf196960
111. Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6r,12ar)-
112. Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6r-trans)-
113. (-)-tadalafil (6r ,12as) Diastereomer
114. Sw197603-2
115. A23556
116. D02008
117. Ab00639969-08
118. Ab00639969_09
119. Ab00639969_10
120. 596t295
121. Ar-270/43507798
122. Q424156
123. Sr-05000001940
124. Sr-05000001940-1
125. Brd-k93645900-001-04-8
126. Tadalafil Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
127. (2r,8r)-2-(1,3-benzodioxol-5-yl)-6-methyl-3,6,17-triazatetracyclo[8.7.0.03,8.011,16]heptadeca-1(10),11,13,15-tetraene-4,7-dione
128. (2r,8r)-2-(2h-1,3-benzodioxol-5-yl)-6-methyl-3,6,17-triazatetracyclo[8.7.0.0^{3,8}.0^{11,16}]heptadeca-1(10),11(16),12,14-tetraene-4,7-dione
129. (2r,8r)-2-(2h-1,3-benzodioxol-5-yl)-6-methyl-3,6,17-triazatetracyclo[8.7.0.0^{3,8}.0^{11,16}]heptadeca-1(10),11,13,15-tetraene-4,7-dione
130. (6r,12 Ar)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
131. (6r,12ar) 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino [1',2':1,6] Pyrido[3,4-b]indole-1,4-dione
132. (6r,12ar) 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
133. (6r,12ar)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methylpyrazino[1,2:1,6]pyrido[3,4-b]indole-1,4-dione
134. (6r,12ar)-6-(1,3-benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1,2,1,6]pyrido[3,4-b]indole-1,4-dione
135. (6r,3,6,7,12,12a-hexahydro-2-methyl-6-[3,4-(methylenedioxy)phenyl]pyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione
136. 1242099-07-5
137. 171488-03-2
138. 6-(1,3-benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione
139. Pyrazino(1',2':1,6)pyrido(3,4-b)indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6r-12ar)-
140. Pyrazino[1',6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12ahexahydro-2-methyl-, (6r,12ar)-
141. Rel-(6r,12ar)-6-(benzo[d][1,3]dioxol-5-yl)-2-methyl-2,3,12,12a-tetrahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4(6h,7h)-dione
| Molecular Weight | 389.4 g/mol |
|---|---|
| Molecular Formula | C22H19N3O4 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 389.13755610 g/mol |
| Monoisotopic Mass | 389.13755610 g/mol |
| Topological Polar Surface Area | 74.9 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 702 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Adcirca |
| PubMed Health | Tadalafil (By mouth) |
| Drug Classes | Antihypertensive, Erectile Dysfunction Agent |
| Drug Label | ADCIRCA (tadalafil), an oral treatment for pulmonary arterial hypertension, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a mo... |
| Active Ingredient | Tadalafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Eli Lilly |
| 2 of 6 | |
|---|---|
| Drug Name | Cialis |
| PubMed Health | Tadalafil (By mouth) |
| Drug Classes | Antihypertensive, Erectile Dysfunction Agent |
| Drug Label | CIALIS (tadalafil) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.41. The structural formula is: The ch... |
| Active Ingredient | Tadalafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Lilly |
| 3 of 6 | |
|---|---|
| Drug Name | Tadalafil |
| PubMed Health | Tadalafil (By mouth) |
| Drug Classes | Antihypertensive, Erectile Dysfunction Agent |
| Drug Label | CIALIS (tadalafil) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.41. The structural formula is: The ch... |
| Active Ingredient | Tadalafil |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 20mg |
| Market Status | Tentative Approval |
| Company | Synthon Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Adcirca |
| PubMed Health | Tadalafil (By mouth) |
| Drug Classes | Antihypertensive, Erectile Dysfunction Agent |
| Drug Label | ADCIRCA (tadalafil), an oral treatment for pulmonary arterial hypertension, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a mo... |
| Active Ingredient | Tadalafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Eli Lilly |
| 5 of 6 | |
|---|---|
| Drug Name | Cialis |
| PubMed Health | Tadalafil (By mouth) |
| Drug Classes | Antihypertensive, Erectile Dysfunction Agent |
| Drug Label | CIALIS (tadalafil) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.41. The structural formula is: The ch... |
| Active Ingredient | Tadalafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Lilly |
| 6 of 6 | |
|---|---|
| Drug Name | Tadalafil |
| PubMed Health | Tadalafil (By mouth) |
| Drug Classes | Antihypertensive, Erectile Dysfunction Agent |
| Drug Label | CIALIS (tadalafil) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.41. The structural formula is: The ch... |
| Active Ingredient | Tadalafil |
| Dosage Form | Tablet |
| Route | oral |
| Strength | 20mg |
| Market Status | Tentative Approval |
| Company | Synthon Pharms |
Tadalafil is indicated for the treatment of erectile dysfunction. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
The Food and Drug Administration ... approved updated labeling for Cialis, Levitra and Viagra to reflect a small number of post-marketing reports of sudden vision loss, attributed to NAION (non arteritic ischemic optic neuropathy), a condition where blood flow is blocked to the optic nerve. FDA advises patients to stop taking these medicines, and call a doctor or healthcare provider right away if they experience sudden or decreased vision loss in one or both eyes. Further, patients taking or considering taking these products should inform their health care professionals if they have ever had severe loss of vision, which might reflect a prior episode of NAION. Such patients are at an increased risk of developing NAION again.
US FDA; FDA Statement. FDA Updates Labeling for Viagra, Cialis and Levitra for Rare Post-Marketing Reports of Eye Problems (July 8, 2005) Available from, as of July 12, 2005: https://www.fda.gov/bbs/topics/NEWS/2005/NEW01201.html
Cardiovascular status of patients should be considered since there is a degree of risk associated with sexual activity; treatments for erectile dysfunction, including tadalafil, should not be used in men for whom sexual activity is inadvisable as a result of their underlying cardiac status.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
The following groups of patients with cardiovascular disease were not included in clinical safety and efficacy trials for Cialis, and, therefore, the use of Cialis is not recommended in these groups until further information is available: patients with a myocardial infarction within the last 90 days, patients with unstable angina or angina occurring during sexual intercourse, patients with New York Heart Association Class 2 or greater heart failure in the last 6 months, patients with uncontrolled arrhythmias, hypotension (<90/50 mm Hg), or uncontrolled hypertension (>170/100 mm Hg), and patients with a stroke within the last 6 months. In addition, patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials, and use in these patients is not recommended.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004.
The effect of a 100 mg single dose of tadalafil on the QT interval was evaluated at the time of peak tadalafil concentration in a randomized, double-blinded, placebo, and active (intravenous ibutilide)-controlled crossover study in 90 healthy males aged 18 to 53 years. The mean change in QTc (Fridericia QT correction) for tadalafil, relative to placebo, was 3.5 milliseconds (two-sided 90% CI=1.9, 5.1). The mean change in QTc (Individual QT correction) for tadalafil, relative to placebo, was 2.8 milliseconds (two-sided 90% CI=1.2, 4.4). A 100 mg dose of tadalafil (5 times the highest recommended dose) was chosen because this dose yields exposures covering those observed upon coadministration of tadalafil with potent CYP3A4 inhibitors or those observed in renal impairment. In this study, the mean increase in heart rate associated with /this/ dose of tadalafil compared to placebo was 3.1 beats per minute.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004.
For more Drug Warnings (Complete) data for TADALAFIL (18 total), please visit the HSDB record page.
Tadalafil is indicated for the treatment of erectile dysfunction (ED) and either alone or in combination with [finasteride] for the treatment of benign prostatic hypertrophy (BPH). It is also indicated for the treatment of pulmonary arterial hypertension (PAH) both alone and in combination with [macitentan] or other endothelin-1 antagonists.
FDA Label
Adcirca is indicated in adults for the treatment of pulmonary arterial hypertension (PAH) classified as World Health Organization functional class II and III, to improve exercise capacity (see section 5. 1).
Efficacy has been shown in idiopathic PAH (IPAH) and in PAH related to collagen vascular disease.
Treatment of erectile dysfunction.
In order for tadalafil to be effective, sexual stimulation is required.
Cialis is not indicated for use by women.
Treatment of erectile dysfunction in adult males.
In order for tadalafil to be effective, sexual stimulation is required.
Tadalafil Lilly is not indicated for use by women.
Treatment of the signs and symptoms of benign prostatic hyperplasia in adult males.
Treatment of erectile dysfunction in adult males.
In order for tadalafil to be effective, sexual stimulation is required.
Tadalafil Mylan is not indicated for use by women.
Talmanco is indicated in adults for the treatment of pulmonary arterial hypertension (PAH) classified as WHO functional class II and III, to improve exercise capacity. Efficacy has been shown in idiopathic PAH (IPAH) and in PAH related to collagen vascular disease.
Treatment of pulmonary arterial hypertension
Tadalafil exerts a therapeutic effect in ED by increasing sexual stimulation-dependant smooth muscle relaxation in the penis, allowing the corpus cavernosum to fill with blood to produce an erection. Smooth muscle relaxation in the pulmonary vasculature helps to produce vasodilation in PAH which reduces blood pressure in the pulmonary arteries. In BPH, tadalafil may contribute to decreased smooth muscle cell proliferation which may reduce the size of the prostate and relieve the anatomical obstruction which produces urinary symptoms of BPH. The decreased affinity of tadalafil for PDE6 compared to other PDE5 inhibitors may explain the reduced incidence of visual side effects.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Phosphodiesterase 5 Inhibitors
Compounds that specifically inhibit PHOSPHODIESTERASE 5. (See all compounds classified as Phosphodiesterase 5 Inhibitors.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
G04BE08
G04BE08
G04BE08
G04BE08
G04BE08
G04BE08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BE - Drugs used in erectile dysfunction
G04BE08 - Tadalafil
Absorption
Tadalafil has a tmax of 0.5-6h with a median of 2h in healthy adults. The tmax in adults with PAH is reported as 2-8h with a median of 4h. There does not appear to be a significant effect on absorption when tadalafil is taken with food.
Route of Elimination
Tadalafil is primarily eliminated via hepatic metabolism. These metabolites are mainly excreted in the feces (61%) and to a lesser extent in the urine (36%)
Volume of Distribution
Tadalafil has a mean apparent volume of distribution of 63L in healthy adults. The mean apparent volume of distribution is reported as 77L in adults with PAH.
Clearance
The mean apparent oral clearance of tadalafil is 2.5-3.4L/h in healthy adults. The mean apparent oral clearance in adults with PAH is reported as 3.5L/h
Tmax: 30 minutes to 6 hours (median 2 hours). Absolute bioavailability has not been determined; rate and extent of absorption are not influenced by food.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
Volume of distribution: 63 L; indicating distribution into tissues. Less than 0.0005% of administered dose was found in the semen of healthy subjects. 94% protein bound.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
Over a dose range of 2.5 to 20 mg, tadalafil exposure (AUC) increases proportionally with dose in healthy subjects. Steady-state plasma concentrations are attained within 5 days of once-daily dosing, and exposure is approximately 1.6-fold greater than after a single dose.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004.
Elimination: Mean oral clearance: 2.5 L per hour. Fecal: 61%. Urine: 36%.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
For more Absorption, Distribution and Excretion (Complete) data for TADALAFIL (11 total), please visit the HSDB record page.
Tadalafil undergoes hepatic metabolism via CYP3A4 to a catechol metabolite. This catechol metabolite undergoes subsequent methylation and glucuronidation with the methyl-glucuronide metabolite becoming the primary metabolite in circulation. None of the known metabolites are considered to be active.
Biotransformation: Hepatic metabolism, mainly by CYP3A4. Tadalafil is predominantly metabolized by CYP3A4 to a catechol metabolite. The catechol metabolite undergoes extensive methylation and glucuronidation to form the methylcatechol and methylcatechol glucuronide conjugate, respectively. The major circulating metabolite is the methylcatechol glucuronide. Methylcatechol concentrations are less than 10% of glucuronide concentrations. In vitro data suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
The mean half-life of elimination of tadalafil is 15-17.5h in healthy adults. The mean half-life of elimination in adults with PAH is reported as 35h.
Terminal: 17.5 hours
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
Tadalafil is a selective phosphodiesterase-5 (PDE5) inhibitor that produces several downstream effects with the most common therapeutic effect being smooth muscle relaxation. Patients may experience ED due to a variety of causes including psychogenic, neurogenic, vasculogenic, iatrogenic, or endocrine. These causes result in dysfunction of penile smooth muscle relaxation through either disrupted neuronal signaling or direct influence on smooth muscle cells. During sexual arousal, non-adrenergic non-cholinergic (NANC) neurons release nitric oxide (NO). Nitric oxide stimulates guanylate cyclase which converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP). cGMP activates the cGMP-dependent kinase (PKG) in a signal cascade which activates K+ channels leading to inhibition of Ca2+ channels, inhibits platelet activation, and inhibits smooth muscle cell proliferation while inducing apoptosis. This signal cascade is attenuated by PDE5 which breaks the phosphodiester bond of cGMP, converting it to GMP. Inhibition of PDE5 by tadalafil increases signaling via the PKG cascade which supports penile smooth muscle relaxation during sexual arousal by decreasing Ca2+ entry into smooth muscle cells. This smooth muscle relaxation allows blood to fill the corpus cavernosum thereby producing an erection. In PAH, blood pressure in the pulmonary arteries is raised due to a variety of mechanisms stemming from endothelial dysfunction. Decreased production of NO and prostacyclin reduce vasodilatory signaling while overproduction of endothelin-1 and thromboxane increase vasoconstriction. Inflammation, thromboses, and hypoxia later contribute to vascular remodeling which further reduces luminal size. The resultant increase in blood pressure reduces the capacity for gas exchange and increases afterload at the right ventricle, producing symptoms of dyspnea, fatigue, and dizziness as well as leading to right-sided heart failure. Tadalafil exerts its therapeutic effect in PAH through boosting NO-cGMP signaling to contribute to smooth muscle relaxation as with ED. Lastly, tadalafil is used to treat BPH. BPH produces urinary dysfunction through hyperproliferation of the epithelial and smooth muscle layers of the prostate. The increased size of the prostate blocks urine flow through the urethra resulting in higher residual volumes due to incomplete emptying. Tadalafil does not appear to exert its benefit via smooth muscle relaxation of the prostate. It may instead exert its effect through a mix of increased oxygenation and decreased inflammation, which decreases tissue remodeling, and inhibition of cell proliferation through the cGMP cascade. The decreased affinity for PDE6 compared to other PDE5 inhibitors may explain the decreased incidence of visual side effects as PDE6 is present in the eye and contributes to color vision.
In vitro studies have shown that the effect of tadalafil is more potent on phosphodiesterase type 5 (PDE5) than on other phosphodiesterases. These studies have shown that tadalafil is >10,000-fold more potent for PDE5 than for PDE1, PDE2, PDE4, and PDE7 enzymes, which are found in the heart, brain, blood vessels, liver, leukocytes, skeletal muscle, and other organs. Tadalafil is >10,000-fold more potent for PDE5 than for PDE3, an enzyme found in the heart and blood vessels. Additionally, tadalafil is 700-fold more potent for PDE5 than for PDE8, PDE9, PDE10 and 14-fold more potent for PDE5 than for PDE11A1, an enzyme found in human skeletal muscle. Tadalafil inhibits human recombinant PDE11A1 activity at concentrations within the therapeutic range. The physiological role and clinical consequence of PDE11 inhibition in humans have not been defined.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
Studies in vitro have demonstrated that tadalafil is a selective inhibitor of phosphodiesterase type 5 (PDE5). PDE5 is found in corpus cavernosum smooth muscle, vascular and visceral smooth muscle, skeletal muscle, platelets, kidney, lung, cerebellum, and pancreas.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosum smooth muscle. This response is mediated by the release of nitric oxide (NO) from nerve terminals and endothelial cells, which stimulates the synthesis of cyclic GMP in smooth muscle cells. Cyclic GMP causes smooth muscle relaxation and increased blood flow into the corpus cavernosum. The inhibition of phosphodiesterase type 5 (PDE5) enhances erectile function by increasing the amount of cyclic GMP. Tadalafil inhibits PDE5. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 by tadalafil has no effect on the absence of sexual stimulation.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.

Certificate Number : R1-CEP 2015-101 - Rev 00
Issue Date : 2021-04-30
Type : Chemical
Substance Number : 2606
Status : Valid

Registration Number : 230MF10089
Registrant's Address : Joan Buscala, 10 E-08173 Sant Cugat del Valles, Barcelona, Spain
Initial Date of Registration : 2018-07-20
Latest Date of Registration : --

NDC Package Code : 64552-4047
Start Marketing Date : 2008-01-07
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.

GDUFA
DMF Review : Reviewed
Rev. Date : 2018-03-07
Pay. Date : 2017-11-15
DMF Number : 24590
Submission : 2011-01-31
Status : Active
Type : II

Certificate Number : CEP 2012-150 - Rev 02
Issue Date : 2023-10-24
Type : Chemical
Substance Number : 2606
Status : Valid

Registration Number : 302MF10011
Registrant's Address : 19 Pellinska Str. 83-200 Starogard Gdanski POLAND
Initial Date of Registration : 2020-01-24
Latest Date of Registration : --

| Available Reg Filing : ASMF, CN |
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.
Shuangda Pharmaceutical has a world-class first-class production line, having GMP, FDA and other regulatory certifications.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37995
Submission : 2023-02-10
Status : Active
Type : II

Certificate Number : CEP 2014-339 - Rev 03
Issue Date : 2024-02-14
Type : Chemical
Substance Number : 2606
Status : Valid

Date of Issue : 2022-08-11
Valid Till : 2025-06-26
Written Confirmation Number : WC-0054
Address of the Firm :

Registrant Name : Gapi Bio Co., Ltd.
Registration Date : 2019-10-14
Registration Number : 20191014-203-I-583-16
Manufacturer Name : Ami Lifesciences Private Limited
Manufacturer Address : Block No. 82/B, ECP Road At & Post. Karakhadi, Tal-Padra, City : Karakhadi-391450, Dist : Vadodara, Gujarat State India

| Available Reg Filing : MX, CN |
 Century has been an API manufacturer for over 30 years & is the partner of choice for multipurpose custom manufacturing projects.
Century has been an API manufacturer for over 30 years & is the partner of choice for multipurpose custom manufacturing projects.
 Hubei Gedian Humanwell focuses on R&D, production, & sales of fertility regulation drugs & steroidal APIs.
Hubei Gedian Humanwell focuses on R&D, production, & sales of fertility regulation drugs & steroidal APIs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?