
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
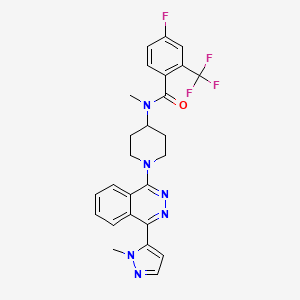

1. Ly2940680
2. Taledegib
1. 1258861-20-9
2. Ly2940680
3. Ly-2940680
4. 4-fluoro-n-methyl-n-(1-(4-(1-methyl-1h-pyrazol-5-yl)phthalazin-1-yl)piperidin-4-yl)-2-(trifluoromethyl)benzamide
5. Ly 2940680
6. Qy8bwx1lj5
7. Taladegib (ly2940680)
8. 4-fluoro-n-methyl-n-[1-[4-(2-methylpyrazol-3-yl)phthalazin-1-yl]piperidin-4-yl]-2-(trifluoromethyl)benzamide
9. 4-fluoro-n-methyl-n-{1-[4-(1-methyl-1h-pyrazol-5-yl)phthalazin-1-yl]piperidin-4-yl}-2-(trifluoromethyl)benzamide
10. Benzamide, 4-fluoro-n-methyl-n-(1-(4-(1-methyl-1h-pyrazol-5-yl)-1-phthalazinyl)-4-piperidinyl)-2-(trifluoromethyl)-
11. 4-fluoro-n-methyl-n-(1-(4-(1-methyl-1h-pyrazol-5-yl)-1-phthalazinyl)-4-piperidinyl)-2-(trifluoromethyl)benzamide
12. Benzamide, 4-fluoro-n-methyl-n-[1-[4-(1-methyl-1h-pyrazol-5-yl)-1-phthalazinyl]-4-piperidinyl]-2-(trifluoromethyl)-
13. Taladegib [usan:inn]
14. Unii-qy8bwx1lj5
15. Taladegib [inn]
16. 4-fluoro-n-methyl-n-{1-[4-(1-methyl-1h-pyrazol-5-yl)-1-phthalazinyl]-4-piperidinyl}-2-(trifluoromethyl)benzamide
17. Taladegib (usan/inn)
18. Taladegib [usan]
19. Taladegib [who-dd]
20. Mls006011066
21. Ly-2940680(taladegib)
22. Schembl2128615
23. Chembl2142592
24. Gtpl10333
25. Dtxsid50154986
26. Ex-a156
27. Bcp02512
28. Bdbm50545020
29. Mfcd21609264
30. Nsc767896
31. S2157
32. Zinc68247898
33. Akos026674116
34. Bcp9000881
35. Ccg-269788
36. Cs-0459
37. Db12550
38. Nsc-767896
39. Sb16504
40. Ncgc00263170-01
41. Ncgc00263170-06
42. Ac-33096
43. As-75020
44. Hy-13242
45. Smr004702857
46. D10671
47. J-515412
48. Q27287564
49. 1ks
| Molecular Weight | 512.5 g/mol |
|---|---|
| Molecular Formula | C26H24F4N6O |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 4 |
| Exact Mass | 512.19477206 g/mol |
| Monoisotopic Mass | 512.19477206 g/mol |
| Topological Polar Surface Area | 67.2 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 794 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?